Abstract: Even though natural health professionals agree that humans should not try to consume industrial chemicals, most seem to overlook this fact when mineral supplementation is involved. And even though many people interested in natural health take minerals, the truth is that nearly all the minerals taken are “natural” for nothing except plants and/or industrial chemicals. While plants are designed to ingest and break-down minerals, humans are not. The truth about nearly all minerals in supplements is that they are really industrial chemicals made from processing rocks with one or more acids. The consumption of this “other half” of the mineral compound is not only unnatural, it can lead to toxicity concerns. Humans were designed to eat food and to get their minerals from foods. Foods DO NOT naturally contain minerals bound to substances such as picolinic acid, carbonates, oxides, phosphates, etc. When supplementation is indicated, only supplements made from 100% food should be considered for supporting optimal health.
In a nutritional context, minerals are certain elements, such as iron and phosphorus that are essential for the physiology of living organisms to exist.
When it comes to nutrition, plants and humans differ: “a typical plant makes its own food from raw materials… A typical animal eats its food” [1]. For plants, these raw materials include soil-based inorganic mineral salts [2]. Soil-based mineral salts can be depleted through synthetic fertilizers, herbicides, pesticides, as well as repeatedly growing crops on the same soil [3,4].
Plants, with the aid of enzymes and soil-based microorganisms, can take in from soil the mineral salts that they have an affinity for through their roots or hyphae [4]. After various metabolic processes, when these minerals no longer exist as salts, they become complexed with various carbohydrates, lipids, and proteins present in the plant as part of the living organism [5]. Thus for nutrition, humans eat plants and/or animals that eat plants, whereas plants can obtain their nutrients from the soil [4]. This process is commonly referred to as the “food chain” [5].
Unfortunately most mineral supplements contain minerals in the form referred to as ‘mineral salts’. Even though mineral salts are often called “natural”, they are rocks (e.g. calcium carbonate exists as the rock commonly known as limestone) or they are chemically produced in accordance with the United States Pharmacopoeia (USP). Mineral salts are natural food for plants, they are not a natural food for humans–humans do not have roots or hyphae!
Dietary Guideline number 18 of the Weston A. Price Foundation, an organization devoted to consuming real foods, is: “Use only natural, food-based supplements” [6]. One of the standards of naturopathy agreed to in 1947 was, “Naturopathy does not make use of synthetic or inorganic vitamins or minerals” [7]. Why would naturopaths have mentioned minerals since they are ‘natural’? Because even back then, most naturopaths knew that the inorganic minerals being placed into supplements were often simply industrial rocks and not foods. Little has changed in the nearly seven decades since. This paper documents the availability, sources, and some of the chemical differences between minerals found in foods and the industrially processed mineral salts that are found in most ‘natural’ mineral supplements.
Absorption
Mineral absorption is affected by many factors including the chemical form, structural form, existence or lack of protein chaperones, health, dietary factors, and even medications.
“Absorptive efficiency for many minerals is governed by homeostatic feedback regulation. When the body is in a depleted state, the intestine upregulates absorption of the nutrient. At the biochemical level , this regulation must be expressed by the control of intraluminal binding lignans, cell-surface receptors, intracellular carrier proteins, intracellular storage proteins, or the energetics of the transmembrane transport…In general mineral bioavailability decreases because of many drugs, decreases with age, and in the presence of malnutrition, is associated with poorer integrity of the small intestine. Therefore, older individuals who are often taking numerous medications and who are eating more poorly than young people are at greater risk of mineral deficiencies” [8].
Chemical Differences
The basic difference between minerals found in foods and those found in industrial mineral salts is chemical.
“The chemical form of a mineral is an important factor in its absorption and bioavailability…there is evidence that the form in which minerals are ingested affects absorption. For example, particle size, surface area, and solubility of a substance affects is dilution rate…In many solid foods, elements are not free, but firmly bound in the food matrix” [8].
This, of course, is not true of most minerals in supplements as they are normally industrially processed inorganic rocks (mineral salts) hence they are void of the factors found in a food matrix. Only 100% food minerals have minerals attached in a food matrix.
Minerals are normally found in food and in the body they are attached with some peptide [9,10]. When humans eat plants or animals they are consuming minerals in those forms. Humans are not supposed to directly consume soil components [1]. With the exception of sodium chloride (common table salt), humans do not normally in any significant quantity consume minerals in the chemical forms known as mineral salts. When they do, it is considered to be a disorder called ‘geophagia’ or ‘pica’ [11,12].
It is a fact that mineral salts are often called “natural”, but they are not food minerals. Mineral salts are normally inorganic molecular compounds that look like rocks [13]. Mineral salts are a compound containing a mineral element, which is the mineral normally listed on a supplement label, and some other substance it is chemically bound to. Mineral salts are either rocks (e.g. calcium carbonate exists as the rock commonly known as limestone) or they are rocks that are chemically-altered. Mineral salts are natural food for plants which can chemically change and detoxify them [14]; they are not a natural food for humans, although some people do consider crushed bones and naturally-calcified sea algae, etc. as food. Minerals bound in mineral salts simply are not treated the same way in the body as are minerals found in food.
Minerals vs. Industrial Chemicals
The following list describes what many mineral salts/chelates used in supplements actually are and what they are used for when not in supplements:
- Boric acid is the rock known as sassolite. It is used in weatherproofing wood, fireproofing fabrics, and as an insecticide [15].
- Calcium ascorbate is calcium carbonate processed with ascorbic acid and acetone. It is a manufactured product used in ‘non-food’ supplements [15].
- Calcium carbonate is the rock known as limestone or chalk. It is used in the manufacture of paint, rubber, plastics, ceramics, putty, polishes, insecticides, and inks. It is also used in fillers for adhesives, matches, pencils, crayons, linoleum, insulating compounds, and welding rods [15].
- Calcium chloride is calcium carbonate and chlorine and is the by product of the Solvay ammonia-soda process. It is used for antifreeze, refrigeration, fire extinguisher fluids, and to preserve wood and stone. Other uses include cement, coagulant in rubber manufacturing, controlling dust on unpaved roads, freezeproofing of coal, and increasing traction in tires [15].
- Calcium citrate is calcium carbonate processed with lactic and citric acids. It is used to alter the baking properties of flour [15].
- Calcium gluconate is calcium carbonate processed with gluconic acid, which is used in cleaning compounds. It is used in sewage purification and to prevent coffee powders from caking [15].
- Calcium glycerophosphate is calcium carbonate processed with dl-alpha-glycerophosphates. It is used in dentifrices, baking powder, and as a food stabilizer [15].
- Calcium hydroxyapatite is crushed bone and bone marrow. It is used as a fertilizer [16].
- Calcium iodide is calcium carbonate processed with iodine. It is an expectorant [15].
- Calcium lactate is calcium carbonate processed with lactic acid. It is used as a dentifrice and as a preservative [15].
- Calcium oxide is basically burnt calcium carbonate. It is used in bricks, plaster, mortar, stucco, and other building materials. It is also used in insecticides and fungicides [15].
- Calcium phosphate, tribasic is the rock known as oxydapatit or bone ash. It is used in the manufacture of fertilizers, milk-glass, polishing powders, porcelain, pottery, and enamels [15].
- Calcium stearate is an octodecanoic calcium salt and can be extracted from animal fat. It is used for waterproofing fabrics and in the production of cement, stucco, and explosives [15].
- Chromium chloride is a preparation of hexahydrates. It is used as a corrosion inhibitor and waterproofing agent [15].
- Chromium picolinate is chromium III processed with picolinic acid. Picolinic acid is used in herbicides [17].
- Copper aspartate is made “from the reaction between cupric carbonate and aspartic acid (from chemical synthesis)” [18]. It is a manufactured product used in ‘non-food’ supplements [18].
- Copper (cupric) carbonate is the rock known as malachite. It is used as a paint and varnish pigment, plus as a seed fungicide [15].
- Copper gluconate is copper carbonate processed with gluconic acid. It is used as a deodorant [19].
- Copper (cupric) glycinate is a copper salt processed with glycine. It is used in photometric analysis for copper [15].
- Copper sulfate is copper combined with sulfuric acid. It is used as a drain cleaner and to induce vomiting; it is considered as hazardous heavy metal by the City of Lubbock, Texas that “can contaminate our water supply” [20].
- Dicalcium phosphate is the rock known as monetite, but can be made from calcium chloride and sodium phosphate. It is used in ‘non-food’ supplements [18].
- Ferric pyrophosphate is an iron rock processed with pyrophosphoric acid. It is used in fireproofing and in pigments [15].
- Ferrous lactate is a preparation from isotonic solutions. It is used in ‘non-food’ supplements [15].
- Ferrous sulfate is the rock known as melanterite. It is used as a fertilizer, wood preservative, weed-killer, and pesticide [15].
- Magnesium carbonate is the rock known as magnesite. It is used as an antacid, laxative, and cathartic [15].
- Magnesium chloride is magnesium ammonium chloride processed with hydrochloric acid. It fireproofs wood, carbonizes wool, and is used as a glue additive and cement ingredient [15].
- Magnesium citrate is magnesium carbonate processed with acids. It is used as a cathartic [15].
- Magnesium glycinate is a magnesium salt processed with glycine. It is used in ‘non-food’ supplements.
- Magnesium oxide is normally burnt magnesium carbonate. It is used as an antacid and laxative [15].
- Manganese carbonate is the rock known as rhodochrosite. It is used as a whitener and to dry varnish [15].
- Manganese gluconate is manganese carbonate or dioxide processed with gluconic acid. It is a manufactured item used in ‘non-food’ supplements [15].
- Manganese sulfate is made “from the reaction between manganese oxide and sulfuric acid” [18]. It is used in dyeing and varnish production [15].
- Molybdenum ascorbate is molybdenite processed with ascorbic acid and acetone. It is a manufactured item used ‘non-food’ supplements [21].
- Molybdenum disulfide is the rock known as molybdenite. It is used as a lubricant additive and hydrogenation catalyst [15].
- Potassium chloride is a crystalline substance consisting of potassium and chlorine. It is used in photography [15].
- Potassium iodide is made from HI and KHCO3 by melting in dry hydrogen and undergoing electrolysis. It is used to make photographic emulsions and as an expectorant [15].
- Potassium sulfate appears to be prepared from the elements in liquid ammonia. It is used as a fertilizer and to make glass [15].
- Selenium oxide is made by burning selenium in oxygen or by oxidizing selenium with nitric acid. It is used as a reagent for alkaloids or as an oxidizing agent [15].
- Seleniomethionine is a selenium analog of methionine. It is used as a radioactive imaging agent [15].
- Silicon dioxide is the rock known as agate. It is used to manufacture glass, abrasives, ceramics, enamels, and as a defoaming agent [15].
- Vanadyl sulfate is a blue crystal powder known as vanadium oxysulfate. It is used as a dihydrate in dyeing and printing textiles, to make glass, and to add blue and green glazes to pottery [15].
- Zinc acetate is made from zinc nitrate and acetic anhydride. It is used to induce vomiting [15].
- Zinc carbonate is the rock known as smithsonite or zincspar. It is used to manufacture rubber [15].
- Zinc chloride is a combination of zinc and chlorine. It is used as an embalming material [15].
- Zinc citrate is smithsonite processed with citric acid. It is used in the manufacture of some toothpaste [15].
- Zinc gluconate is a zinc rock processed with gluconic acid. Gluconic acid is used in many cleaning compounds [15].
- Zinc lactate is smithsonite processed with lactic acid. Lactic acid lactate is used as a solvent [15].
- Zinc monomethionine is a zinc salt with methionine. It is used as a ‘non-food’ supplement.
- Zinc orotate is a zinc rock processed with orotic acid. Orotic acid is a uricosuric (promotes uric acid excretion) [15].
- Zinc oxide is the rock known as zincite. It is used as a pigment for white paint and as part of quick-drying cement [15].
- Zinc phosphate is the rock known as hopeite. It is used in dental cements [15].
- Zinc picolinate is a zinc rock processed with picolinic acid. Picolinic acid is used in herbicides [17].
- Zinc sulfate can be a rock processed with sulfuric acid. It is used as a corrosive in calico-printing and to preserve wood [15].
There is a relatively easy way to tell if minerals are industrial chemicals. Whenever there are two-words on a label describing a mineral, it is a logical to conclude that the substance is an industrial mineral product and not 100% food. The exception is chromium GTF (the GTF stands for glucose tolerance factor) which is food if it is from nutritional yeast [18].
Chelated Minerals
Chelated minerals are generally crushed industrial rocks that are processed with one or more acids.
Probably the biggest difference in minerals now compared to 1947 is that some companies have decided to industrially produce versions of minerals attached to peptides. Essentially they take a rock or industrial mineral salt, chemically alter it, and attempt to attach it to the mineral. This results in a mineral that is different from normal mineral salts, but does not turn the substance into a food. Examples of this include the various mineral ascorbates, picolinates, aspartates, glycinates, and chelates. It needs to be understood that since there is not a universally accepted definition of the term ‘chelate’, when this term is used on a label, one generally does not know if the chelate is amino-acid based or some type of industrial acid.
While it is true that humans can, and do, utilize minerals from USP mineral salts or chelated minerals, this is not as safe (or even normally as effective) as consuming them from foods (or in the case of real food supplements, food concentrates).
Non-Food Attachments, Including Some “Chelates,” Are Not Desirable
Is it wise to consume non-food minerals?
Dr. Bernard Jensen, an early 20th century advocate of food-based nutrition, once wrote, “When we take out from foods some certain salt, we are likely to alter the chemicals in those foods. When extracted from food, that certain chemical salt is extracted, may even become a poison. Potash by itself is a poison, whether it comes from a food or from the drugstore. This is also the case with phosphorus. You thereby overtax your system, and your functions must work harder, in order to throw off those inorganic salts or poisons introduced… The chemical elements that build our body must be in biochemical, life-producing form. They must come to us as food, magnetically, electrically alive, grown from the dust of the earth… When we are lacking any element at all, we are lacking more than one element. There is no one who ever lacked just one element. We don’t have a food that contains only one element, such as a carrot entirely of calcium or sprouts totally made of silicon” [22].
It should be noted that the addition of “citric acid and picolinic acid do not appear to enhance zinc absorption” [23]. Chromium picolinate is a human-made substance, created by Gary Evans [24]; it is not a natural food. Picolinic acid is used in herbicides [17]; furthermore “picolinic acid is an excretory or waste product. It is not metabolized by or useful to the body” [25]. Scientists report, “some research groups recently suggested that chromium (III) picolinate produces significantly more oxidative stress and potential DNA damage than other chromium supplements” [26].
Concerns are being raised from various sources about the implications of intentional ingestion of inorganic substances in supplements by human beings [22,25,26]. These substances are not natural for humans to consume and a long period of consumption may cause some type of toxic accumulation [22,25,26]. Yet, many people supposedly interested in natural health are daily consuming various carbonates, gluconates, oxides, picolinates, phosphates, sulfates and other rock components that were not intended to be ingested that way. Since there are many possible negative implications associated with “the other half” of these non-food minerals [25], people truly interested in their health would be much better off consuming foods that are high in minerals or supplements made from those foods.
Jay Patrick claims to have originally developed procedures to manufacture all seven of the mineral ascorbates [21]; thus it would seem highly inappropriate to call supplements with ascorbate attached minerals ‘food’.
Actually, it does not appear that any of the minerals marketed as ‘chelated’ are food concentrates, though there are foods which contain naturally chelated minerals, but these are normally marketed as food minerals. Even though there are some theoretical advantages to industrially-produced mineral ‘chelates’ as compared to inorganic mineral salts, these chelates are not natural food.
More on Bioavailability
It is well known among nutrition researchers that most essential minerals are not well absorbed; for some minerals, absorption is less than 1% [27]. “Bioavailability of orally administered vitamins, minerals, and trace elements is subject to a complex set of influences…In nutrition science the term ‘bioavailability’ encompasses the sum of impacts that may reduce or foster the metabolic utilization of a nutrient” [28]. Research demonstrates that the bioavailability and/or effectiveness of mineral containing foods is greater than that of isolated inorganic mineral salts or mineral chelates [e.g. 28-52]. These studies have concluded that natural food minerals may be better absorbed, utilized, and/or retained than mineral salts.
Furthermore, minerals used in most supplements do not contain protein chaperones or other food factors needed for absorption into the cell. In 1999, the Nobel Prize for medicine was awarded to Guenter Blobel who discovered that minerals need protein chaperones to be absorbed into cellular receptors. When mineral salts without protein chaperones are consumed, “It is after digestion when other mineral forms {mineral salts} have their mineral cleaved from their carriers. In this situation, these minerals become charged ions, and their absorbability becomes in jeopardy. These charged free minerals are known to block the absorption of one another, or to combine with other dietary factors to form compounds that are unabsorbable” [53]. The body must discard the residual chemicals.
Foods used in supplements that commonly provide significant quantities of essential minerals include dulse, horsetail herb, kelp, nutritional yeast, rice bran, and water thyme. These types of foods have been shown to contain not only minerals in natural food forms, but also important protein chaperones such as ATX1 and ceruplasmin [54,55]. Industrial mineral salts do not contain the protein chaperones or other food factors needed for proper mineral absorption. Furthermore, some foods also contain factors which reduce the probability of certain minerals to be toxic to the body [32,33,55]; industrial mineral salts and chelates are simply not that complete.
Quantitative and Qualitative Differences
There are quantitative and qualitative differences in food vs. non-food minerals. Table 1 lists some of them by mineral.
Table 1 Quantitative and Qualitative Differences
Food Mineral | | Compared to Mineral Salt/Chelate |
Calcium | | Up to 8.79 times more absorbed into the blood [47] and 7 times as effective in raising serum ionic calcium levels [30]. |
Chromium | | Up to 25 times more bioavailable [31]. |
Copper | | 85% more absorbed [45]; also contains substances that reduce potential toxicity [32,46]. |
Iron | | Safer, non-constipating, 77% more absorbed [33, 34, 45]. |
Magnesium | | Up to 2.20 times better absorbed [52] and retained [35]. |
Manganese | | Better absorbed and retained [45,46] and not as likely to contribute to toxicity as mined forms [36,56]. |
Molybdenum | | Up 6.28 times better absorbed into the blood and 16.49 times better retained [45]. |
Phosphorus | | Less likely to cause diarrhea or electrolyte disorders [37]. |
Selenium | | 17.6 time the antioxidant effect [46], 123.01 times more effective in preventing nonenzymatic protein glycation [17], and 2.26 times better retained [29,38,44]. |
Vanadium | | Safer and 50% more effective [39]. |
| Zinc | | Up to 6.46 times better absorbed [45,46,51], better form [40,41]. |
Foods, almost by definition, are not toxic, and as mentioned earlier, can have protective factors to prevent certain potential mineral toxicities, such as those sometimes associated with copper, iron, manganese, or other minerals [32,33,55,56].
Information by Individual Mineral
Some differences between food complexed minerals and mineral salts have been documented by published research and are shown by individual mineral below:
Boron “Boron complexes with organic compounds containing hydroxyl groups” [9], which is how it is found in foods. Boron affects macromineral and steriodal hormone metabolism; without sufficient boron bone composition, strength, and structure weaken [9].
Calcium “The amount of calcium absorbed depends on its interaction with other dietary constituents…The absorbability of calcium is mainly determined by the presence of other food constituents” [56]. This is one of the reasons why isolated calcium mineral salts (such as calcium carbonate) are not absorbed as well as calcium found in natural food complexes [56,57]. “Calcium carbonate, an antacid, counteracts not only the absorption of calcium, but also the absorption of iron” [11] (though its calcium absorption appears to be better with food [58]). At least one researcher has concluded that commonly used mineral salts such as calcium lactate and calcium gluconate primarily succeed in creating high blood calcium levels (hypercalcemia) instead of alleviating symptoms of low tissue calcium [59]. “Calcium has a structural role in bones and teeth” as well as in some enzymes involved with blood clotting [48]. Calcium can affect mood and blood pressure [57,60]. Clinical reports consistently confirm that dietary/food calciums [5-8] are important in the management of blood pressure. This does not appear to be the case with isolated calcium salts (the results appear inconsistent [30,61-63]). One study found that calcium in Food raised serum ionic calcium levels from 1.08 to 1.15 mmoles, but that serum ionic calcium levels were not raised with calcium carbonate [30]. Serum calcium levels affect blood pressure [60,64]. Since low bone mass is somewhat inversely correlated with high levels of diastolic blood pressure [9], this suggests that calcium from Food may be superior when hypertension issues are present. Calcium is important for optimal health as calcium deficiencies can contribute to osteoporosis, muscle cramps (especially in the legs), insomnia, mood/behavioral/nerve problems, hypertension, kidney stones, and colon cancer [61,65,66]. It appears that overdose of calcium can only occur when taking mineral salt forms of calcium supplement as opposed to food [66]. A human study found that Natural Food Complex calcium is 8.79 times more bioavailable than calcium carbonate (which is the most common form found in supplements) and 2.97 times more than calcium gluconate [47]. This same study found that Food calcium “produced no undesirable side effects and was the most suitable form of calcium for long-term supplementation” [47].
Chromium, GTF “The biologically active form of chromium, sometimes called glucose tolerance factor or GTF, has been proposed to be a complex of chromium, nicotinic acid, and possibly the amino acids glycine, cysteine, and glutamic acid. Many attempts have been made to isolate or synthesize the glucose tolerance factor; none have been successful” [67]. Chromium is not naturally found in the body in the commonly supplemented forms such as chromium picolinate or chromium chelate. “Chromium is generally accepted as an essential nutrient that potentiates insulin action, and thus influences carbohydrate, lipid, and protein metabolism” [67]. Research suggests that there is much less likelihood of toxicity from natural food complex chromium than from forms such as chromium picolinate [26]. Only 1% or less of inorganic chromium is absorbed vs.10-25% of chromium GTF [31]. One small study found that Food chromium GTF reduced blood glucose levels by 16.8% versus 6.0% for inorganic chromium [48], thus it was 2.80 times more effective. One study found that Food chromium benefited certain diabetics by improving blood glucose control, lowering serum lipids, and decreasing the risk of coronary heart disease [49]. Chromium GTF only comes from nutritional yeast [58].
Copper In the human body, in addition to various plasma-bound coppers, “at least one copper peptide complex” has been isolated [60]. Copper is predominantly found in Food nutrients in a copper peptide complex (such as Cu/Zn superoxide-dismutase). Copper is not naturally found in the body in the form of copper gluconate or copper sulfate. “Anemia, neutropenia, and osteoporosis are observed with copper deficiency”; copper is involved in connective tissue, iron metabolism, the central nervous system, melanin pigment, thermal regulation, cholesterol metabolism, immune function, and cardiac function [60]. Copper in foods like nutritional yeast contains protective factors that reduce the possibility of toxicity issues [32,46]. A human study found that Food copper was 1.44 times more absorbed into the blood than copper sulfate and 1.43 times more than copper gluconate [45]. Animal studies showed similar results, plus concluded that Food copper was retained in the liver 1.85 times more than copper gluconate and 1.42 times more than copper sulfate [45].
Iodine Most of the iodine in the body exists in the form of iodine-containing amino acids [61]. Iodine is needed by the thyroid gland to produce thyroid hormones which influence most of the body’s metabolic processes [61]. Kelp is an excellent food source of iodine [61].
Iron Most researchers acknowledge that organic iron is better absorbed than inorganic iron [71]. The body has different mechanisms for the absorption of iron depending upon its form [72]. Iron in foods is found in an organic form. Iron is required for growth and hemoglobin formation; inadequate amounts can lead to “weakness, fatigue, pallor, dyspnea on exertion, palpitation, and a sense of being overly tired” [72]. Iron in food is safer, less-constipating (actually it is non-constipating), and better absorbed than non-food forms [33,34]. An animal study found that Food iron was absorbed into the blood 1.01 times more than ferrous sulfate and 1.77 times more than amino acid chelated iron and was retained in the liver 1.21 times more than ferrous sulfate and 1.68 times more than amino acid chelated iron [45,46].
Magnesium “The percentage of absorption of ingested magnesium is influenced by its dietary concentration and by the presence of inhibiting or promoting dietary components [73]. There are no promoting dietary components in inorganic isolated magnesium salts. “Magnesium is involved in many enzymatic steps in which components of food are metabolized and new products are formed”; it is involved in over 300 such reactions [6]. Clinical deficiency of magnesium can results in “depressed tendon reflexes, muscle fasciculations, tremor, muscle spasm, personality changes, anorexia, nausea, and vomiting” [73]. Magnesium in foods is better absorbed and retained than magnesium from inorganic mineral salts [35]. A human study found that Natural Food Complex magnesium was 2.20 times more absorbed into blood than magnesium oxide and 1.60 times more than amino acid chelated magnesium [52].
Manganese In the body, absorbed manganese complexes with various peptides [9]. Manganese is predominantly found in foods in a manganese peptide complex (such as Mn superoxide-dismutase). It is not found in the body in forms like manganese sulfate. Manganese deficiency can cause “impaired growth, skeletal abnormalities, disturbed or depressed reproductive function, ataxia of the newborn, and defects in lipid and carbohydrate metabolism” [9]. It can also affect skin, hair, nails, and problems with calcium metabolism [9]. Manganese in foods is safer and much less likely to cause any toxicity compared to mined forms [36,56]. ]. An animal study found that Natural Food Complex manganese was absorbed 1.56 times more into the blood and was retained 1.63 times more in the liver than manganese sulfate [45,46].
Molybdenum Molybdenum…in foods…is readily absorbed” [9]. “Molydenum in {nearly all} nutritional supplements is in the form of either sodium molybdate or ammonium molybdate. Molybdenum in food is principally in the form of molydenum cofactors” [67]. “Molybdenum functions as an enzyme cofactor”, thus “detoxifies various pyrimidines, purines, pteridines, and related compounds” [9]; it may also affect growth and reproduction [9]. An animal study found that Food molybdenum was absorbed 6.28 times more into the blood and was retained 16.49 times more in the liver than ammonium molybdate and 10.27 times more than molybdenum amino acid chelate [45].
Phosphorus Phosphorus is found in plants [11]. Phosphorus salts can cause diarrhea and other problems [37]—problems that do not happen with phosphorus in foods. Phosphorus works with calcium to produce strong bones [57].
Potassium Potassium is found in plants [11]. Potassium is the leading intracellular electrolyte and is necessary for electrolyte balance, stimulating aldersterone for the adrenal glands, and blood pressure regulation [11]. Dr. Bernard Jensen seemed to believe potassium is only safe in its natural food complex form [22].
Selenium “The predominant form of selenium in animal tissues is selenocysteine” [74]. That is how it is predominantly found in certain foods. One study found that diets naturally high in selenium (daily consumption as high as 724mcg) produced no signs or symptoms of selenium overexposure while another found that exceedingly high consumption of selenium salts could induce selenium poisoning [74]. Selenium seems to support thyroid hormone production, function as part of many enzymes, and have antioxidant effects [74]. Larry Clark, Ph.D. and others have found that selenium in yeast appears to reduce risk of certain cancers [75]. Julian Whitaker, M.D. reports, “The best absorbed form of selenium, and the one used by Dr. Clark’s research, is high-selenium yeast” [75]. A study using 247 mcg/day of high-selenium yeast found that plasma selenium levels were 2-fold higher than baseline values after 3 and 9 months and returned to 136% of baseline after 12 months, whereas there was a 32% increase in blood glutathione levels also seen after 9 months [29]. Food selenium is about twice as well retained as non-food forms [29,38]. Research suggests that Food selenium is 2.26 times more retained in the liver and 1.22 times more absorbed in the blood than sodium selenite [44]. An in vitro study found that Food selenium had 17.6 times the antioxidant effect than did selenomethionine [44]. One study found that Food selenium was 123.01 times more effective than sodium selenite in preventing nonenzymatic glycation in diabetics [50].
Silicon “In animals, silicon is found both free and bound” [9]. Silicon absorption is quite dependent upon the form [9]. Silicon is involved in bone calcification and connective tissue formation [9]. It is also needed for healthy hair and skin [51]. Silicon is found in foods in an organic form.
Trace Minerals Trace minerals, including “ultra trace minerals” are necessary for the proper functioning of human health [9,51]. There are many in the human body, some of which are known to be essential and others of which their “essentialness” is under investigation. Sea vegetables and certain yeasts are a good source of trace minerals [11,31,61].
Vanadium “Vanadate forms compounds with other biological substances” [9]. “Vanadium has been postulated to play a role in the regulation of (NaK)-ATPase, phosphoryl transferase enzymes, adenylate cyclase, and protein kinases; as an enzyme cofactor in the form of vandyl and in hormone, glucose, lipid, and tooth metabolism” [9]. Vanadium in foods is found in an organic form. Vanadium in food is safer than non-food forms and also appears to be about 50% more effective [39].
Zinc Most researchers acknowledge that organic zinc is better absorbed than inorganic zinc [71]. Zinc itself is generally found in the human body in ionic form [71,76]; it is often bound with albumin [23,76] or alpha2-macroglobulin [23] or exists as part of one of the many zinc metalloenzymes [23,76]. Zinc is predominantly found in foods as zinc peptide complex (such as that complexed with superoxide dismutase). Zinc is not naturally found in the body as zinc gluconate, zinc orotate, zinc sulfate, nor zinc picolinate. In humans “zinc deficiency does not exist without deficiency of other nutrients” [76]. Zinc deficiency in humans can cause alopecia, impotence, skin problems, immune deficiencies, night blindness, impaired taste, delayed wound healing, impaired appetite, photophobia, difficulty in dark adaptation, growth retardation, and male infertility [23]. Zinc in yeast-containing foods is better absorbed and is a better form for humans than inorganic forms [40,41]. Studies indicate that Food zinc appears to be 1.72-1.75 times more absorbed in the blood than zinc sulfate (1.71 times more than zinc chelate; 6.46 times more than zinc gluconate; 3.11 times more than zinc orotate) and 1.75-1.87 times more retained in the liver than zinc sulfate (1.45 times more than zinc amino acid chelate; 3.68 times more than zinc gluconate; 1.50 times more than zinc orotate) [45,46,51].
Food and Food Processing
“In the historic struggle for food, humans ate primarily whole foods or so-called natural foods, which underwent little processing…The nutrient content of food usually decreases when it is processed” [77]. “Intensive animal rearing, manipulation of crop production and food processing have altered the qualitative and quantitative balance of nutrients of food consumed by Western society. This change, to which the physiology and biochemistry of man may not be presently adapted to, is thought to be responsible for the chronic diseases that are rampant in the Industrialized Western Countries” [78]. Some reports suggest that simply taking a synthetic multi-vitamin/mineral formula does not change this [79,80].
Dr. Burr-Madsen has written,
Nutrition ‑ in its most basic sense the process by which the organism finds, consumes,liberates, absorbs, and utilizes the nutrients it must have to live. Although food and therefore nutrients are seemingly plentiful, because of modern use of chemical herbicides and pesticides as well as poor air quality and bad water, the nutrients we buy in the market are very inferior. Human bodies require nutrition found in the form of plants, meat, milk, eggs and water, but all animals get their food directly or indirectly from plants, and all plants get their food from the soil. Therefore mineral deficient soil may be one of the greatest original sources of disease in the world today.
Real soil
We cannot appreciate enough the importance of our relationship with the land, with soil. This is particularly so in this era of artificial chemicals, artificial foods, and the abundance of artificial materials on which we have come to depend. This system cannot replace real soil and the living food crops it produces. Our dependence on artificial, manmade products interferes with our relationship with the soil and the natural world in general. Because of this Nutritional supplementation is necessary.
Soil condition.
After genetics and weather, the condition of the soil is the most important factor in thenutrient content of any plant food and, indirectly, of animal foods. The soils of the world have suffered, and continue to suffer, at the hands of farming. The present food production system, while correcting some abuses of the past, inflicts on the soil a variety of new and old insults that diminish its nutrient value. Because of intensive farming, poor crop management, erosion, commercial fertilization, the use of pesticides, and other problematic factors, much of the soil in which our crops are now raised has been depleted, particularly of essential minerals.
The Human Food Chain.
The human food chain includes animals, animal products and plants, which depend directly or indirectly on the soil. Plants draw their nutrients and general health from a complex of inorganic and organic factors. Inorganic substances include oxygen and carbon, nitrogen, phosphorus, and potassium, along with iron, calcium, and an array of other minerals. The chief organic factors range from decaying plant material and animal wastes to earthworms and an amazing variety of microscopic organisms including bacteria, fungi, algae, and protozoa (Hall 1976: 134). All of these elements are important to the health and nutrient value of the crop ‑ and of the animals that feed on it.
Healthy soil.
Healthy soil is America’s greatest natural resource. But few realize that the current state of wide spread soil erosion in North America threatens our way of life. It may be hard to believe, but only a few inches of topsoil stand between you, me, and starvation. We cannot appreciate enough the importance of our relationship with the land, with soil. What is popularly called topsoil is the rich, nutrient‑laden cover of the Earth’s crust from which food crops draw their sustenance. Underneath the topsoil there may be clay, shale, or rock ‑ Substances that do not support food crops. It is only in the precious shallow topsoil that plants are seeded, germinated, sprouted, nurtured, and grown. These plants serve as food for animals on the lowest ends of the food chain. Animals that eat these plants supply food to animals on the highest ends of the food chain. Attention is important because topsoil is easily exhausted from lack of care. The best farmers replenish the soil as it is farmed. Unfortunately, this practice has become an exception to the rule, this is particularly so today.
Depleted Soil.
When the soil becomes depleted, the plants often show symptoms of poor nutrition, much like human deficiency diseases. For example, a general yellow or pale green color (chlorosis) indicates a lack of sulfur and nitrogen and a white or pale‑yellow color iron deficiency. Some of these deficiencies are apparent enough to hurt the marketability of the crop. Most, however, are not visible to the shopper’s or even the farmer’s eye, and the crop is shipped to market deficient as it is. The toll that fertilization and pesticides take on the soil is wide‑reaching, ultimately including the kind of soil erosion that is now plaguing the Midwest. The most direct and immediate loss are the mineral and vitamin deficiencies in the soil that are passed up the food chain to humans (it is a domino effect) [81].
Commercial food processing definitely reduces the nutrient content of food [81, 82] and can be dangerous to human health [83]. The refining of whole grains (including wheat, rice, and corn) has resulted in a dramatic reduction of their natural food complex nutrition [11,82]; specifically the milling of wheat to white flour reduces the natural food complex vitamin and mineral content by 40-60% [82]. Food refining appears to reduce trace minerals such as manganese, zinc, and chromium [2] and various macrominerals (such as magnesium) as well [10,56]. The treatment of canned or frozen vegetables with ethylenediaminetetraacetic acid (EDTA) can strip much of the zinc from foods [11]. The high incidences of disorders of calcium metabolism [28] suggest that the forms of calcium many are consuming simply do not agree with the body (and sometimes result in calcium loss [11]).
Organically-grown produce appears to contain higher levels of some essential minerals than does conventionally (non-organically) grown produce [84,85] and appears to contain lower levels of toxic heavy metals [86]. Even if modern food practices did not affect nutrition (which they do), all minerals that humans need for optimal health do not exist uniformly in soils. “Soils in many areas of the world are deficient in certain minerals; this can result in low concentrations of major or trace minerals in drinking water, plant crops, and even tissues of farm animals, thus contributing to marginal or deficient dietary intakes of humans [76]. From a geological perspective, a few examples include iodine, molybdenum, cobalt, selenium, and boron [2,70,77]. Although humans need at least twenty minerals (over sixty have been found in the body), most plants can be grown with only the addition of nitrogen, phosphorus, and potassium compounds [2]. If other minerals necessary for human health are reduced in the soil, the plant can (and will) grow without them. This means, though, that constantly farming the same ground can result in the reduction of some of the essential minerals we as humans require for optimal health [78].
Ground Up Rocks Pose Risks
Rock minerals are not optimal for human health and post health risks. Perhaps it should be mentioned that typical multi-vitamin-mineral formulas are dangerous and do not result in optimal health. A study involving 38,772 women in the USA who took synthetic multi-vitamins with ground up rock minerals found that the women died earlier than those who did not take them [87]. Other studies have concluded that the acid-processed rocks that many take as calcium supplements increase risk of cardiovascular disease and other problems [88]—yet those studies did not find problems with food calcium.
Ground-up rocks are dangerous to ingest. Yet, 100% food vitamins and minerals are beneficial as well as essential to human health and longevity.
Conclusion
No matter how many industrially produced mineral supplements one takes orally, they will:
1) Never be a truly complete nutrient source.
2) Never replace all the functions of food minerals.
3) Always be unnatural substances to the body.
4) Always strain the body by requiring that it detoxify or somehow dispose of their unnatural structures/chemicals.
5) Never be utilized, absorbed, and retained the same as food nutrients.
6) Not be able to prevent advanced protein glycation end-product formation the same as food nutrients.
7) Never be able to have the antioxidant effects the same as food nutrients.
8) Always be industrial products.
9) Always be composed of petroleum-derivatives, hydrogenated sugars, acids, and/or industrially-processed rocks.
10) Never build optimal health the same as food nutrients.
Industrially processed minerals can have some positive nutritional effects, yet they are not food for humans, but they also pose risks [87-88].
Unlike humans, plants have roots or hyphae which aid in the absorption of minerals. Plants actually have the ability to decrease the toxicity of compounds by changing their biochemical forms [14]. Plants are naturally intended to ingest rocks; humans are not [1].
The truth is that plants, or supplements only made from plants, are the best form of mineral supplement for humans, yet most people who take nutritional mineral support consume some type of industrially processed rock.
REFERENCES
[1] Cronquist A. Plantae. In Synopsis and Classification of Living Organisms, Vol 1. McGraw-Hill, NY, 1982:57
[2] Schroeder HA. The Trace Elements and Man. Devin-Adair, New Greenwich (CT), 1973
[3] Howell E. Enzyme Nutrition. Avery Publishing, Wayne (NJ), 1985
[4] Milne L, Milne M. The Arena of Life: The Dynamics of Ecology. Natural History Press, Garden City (NJ), 1972
[5] Wallace RA. Biology: The World of Life, 6th ed. Harper Collins, New York, 1992
[6] Dietary guidelines in The Weston A. Price Foundation brochure. Weston A. Price Foundation, Washington, 1999
[7] Gehman JM. From the Office of the President: Pseudo-Group Once Again Misleading the Naturopathic Field. Official Bulletin ANA, January 25, 1948:7-8
[8] Shapes SA, Schlussel YR, Cifuentes M. Drug-Nutrient Interactions That Affect Mineral Status. In Handbook of Drug-Nutrient Interactions. Humana Press, Totowa (NJ), 2004: 301-328
[9] Nielsen F. Ultratrace Minerals. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:269-286
[10] Turnland JR. Copper. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:231-241
[11] Whitney EN, Hamilton EMN. Understanding Nutrition, 4ed. West Publishing, New York, 1987
[12] Beers MH, Berkow R, eds. The Merck Manual of Diagnosis and Therapy, 17th ed. Merck Research Laboratories, Whitehouse Station (NJ), 1999
[13] Thiel RJ. Mineral salts are for plants, food complexed minerals are for humans. ANMA Monitor 1999;3(2):5-10
[14] Huang Y, Chen Y, Tao S. Effect of rhizospheric environment of VA-mycorrhizal plants on forms of Cu, Zn, PB and Cd in polluted soil. Ying Yong Sheng Tai Xye Bao 2000;11(3):431-434
[15] Budvari S, et al eds. The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th ed. Merck Research Laboratories, Whitehouse Station (NJ), 1996
[16] Anagisawa KY, Rendon-Angeles JC, Shizawa NI, Ishi SO. Topotaxial replacement of chlorapatite by hydroxy during hydrothermal ion exchange. Am Mineralogist 1999;84:1861-1869
[17] DiTomaso JM. Yellow starthistle: chemical control. Proceedings of the CalEPPC Symposium, 1996, as updated 5/2/02
[18] Vitamin-Mineral Manufacturing Guide Nutrient Empowerment, volume 1. Nutrition Resource, Lakeport (CA), 1986
[19] Hojo Y, Hashimoto I, Miyamoto Y, Kawazoe S, Mizutani T. In vivo toxicity and glutathione, ascorbic acid, and copper level changes induced in mouse liver and kidney by copper (II) gluconate, a nutrient supplement. Yakugaku Zasshi 2000;120(3):311-314
[20] City of Lubbock. www.solidwaste.ci.lubbock.tx.us/hhw/hhw.htm 7/18/02
Cunnane SC. Zinc: Clinical and Biochemical Significance. CRC Press, Boca Raton (FL),1988
[21] Patrick J. What most people don’t know about vitamin C. The Alacer Health Report, Foothill Ranch (CA), 1994
[22] Jensen B. The Chemistry of Man. Bernard Jensen, Escondido (CA),1983
[23] King JC, Keen CL. Zinc. In Modern Nutrition in Health and Disease, 9th ed. Williams & Wilkins, Balt., 1999:223-239
[24] Chromium picolinate, rev. 6/96B.BLI website, July 16, 2002
[25] Implications of the ‘other half’ of a mineral compound. Albion Research Notes 2000;9(3):1-5
[26] Stoecker B.J. Chromium. In Modern Nutrition in Health and Disease, 10th ed. Lippincott Williams & Wilkins, Phil., 2005: 332-337
[27] Turnland JR. Bioavailability of dietary minerals to humans: the stable isotope approach. Crit Rev Food Sci Nutr 1991;30(4);387-396
[28] Schumann K, et al. Bioavailability of oral vitamins, minerals, and trace minerals in perspective. Arzneimittelforshcung 1997;47(4):369-380
[29] El-Bayoumy K, Richie JP Jr, Boyiri T, Komninou D, Prokopczyk B, Trushin N, Kleinman W, Cox J, Pittman B, Colosimo S. Influence of Selenium-Enriched Yeast Supplementation on Biomarkers of Oxidative Damage and Hormone Status in Healthy Adult Males: A Clinical Pilot Study. Cancer Epidemiol Biomarkers Prev. 2002;11:1459-1465
[30] Hamet P, et al. The evaluation of the scientific evidence for a relationship between calcium and hypertension. J Nutr, 1995;125:311S-400S
[31] Ensminger AH, Ensminger ME, Konlade JE, Robson JRK. Food & Nutrition Encyclopedia, 2nd ed. CRC Press, New York, 1993
[32] Avery SV, Howlett NG, Radice S. Copper toxicity towards Saccharomyces cerevisiae: dependence on plasma fatty acid composition. Appl Environ Microbiol 1996;62(11):3960-3966
[33] Wi’snicka R, Krzepiko A, Krawiec Z, Bili’nski T. Protective role of superoxide dismutase in iron toxicity in yeast. Biochem Mol Biol Int 1998;44(3):635-641
[34] Wood R.J., Ronnenberg A.G. Iron. In Modern Nutrition in Health and Disease, 10th ed. Lippincott William & Wilkins, Phil, 2006: 248-270
[35] Rude R.K., Shils M.E. Magnesium. In Modern Nutrition in Health and Disease, 10th ed. Lippincott William & Wilkins, Phil, 2006: 223-247
[36] Buchman A. Manganese. In Modern Nutrition in Health & Disease, 10th ed. Lippincott William & Wilkins, Phil, 2006:326-331
[37] Beloosesky Y, Grinblat J, Weiss A, Grosman B, Gafter U, Chagnac A. Electrolyte disorders following oral sodium phosphate administration for bowel cleansing in elderly patients. Arch Intern Med. 2003;163(7):803-808
[38] Biotechnology in the Feed Industry. Nottingham Press, UK, 1995: 257-267
[39] Badmaev V, Prakash S, Majeed M. Vanadium: a review of its potential role in the fight against diabetes. J Altern Complement Med. 1999;5(3):273-291
[40] Andlid TA, Veide J, Sandberg AS. Metabolism of extracellular inositol hexaphosphate (phytate) by Saccharomyces cerevisiae. Int J. Food Microbiology. 2004;97(2):157-169
[41] King JC, Cousins RJ. Zinc. In Modern Nutrition in Health and Disease, 10th ed. Lipponcott Williams & Wilkins, Phil., 2005:271-285
[42] Thiel R, Fowkes S. Can cognitive deterioration associated with Down syndrome be reduced? Med Hypo, 2005; 64(3):524-532
[43] Jenkins DJA, Wolever TMS, and Jenkins AL. Diet Factors Affecting Nutrient Absorption and Metabolism. In Modern Nutrition in Health and Disease, 8th ed. Lea and Febiger, Phil.:583-602, 1994
[44] Vinson, J.A., Jennifer M. Stella, J.M., Flanagan, T.J. Selenium yeast is an effective in vitro and in vivo antioxidant and hypolipemic agent in normal hamsters. Nutritional Research, 1998, Vol 18, No. 4: 735–742
[45] Vinson J, Bose P, Lemoine L, Hsiao KH. Bioavailability studies. In Nutrient Availability: Chemical and Biological Aspects. Royal Society of Chemistry, Cambridge (UK) 1989:125-127
[46] Vinson JA, Bose P. Comparison of bio-availability of trace elements in inorganic salts, amino acid chelates, and yeast. Mineral Elements 80, Proceedings II, Helsinki, Dec 9-11, 1981
[47] Vinson J, Mazur T, Bose P. Comparisons of different forms of calcium on blood pressure of normotensive males. Nutr Reports Intl, 1987;36(3):497-505
[48] Vinson JA, Hsiao, KH. Comparative effect of various forms of chromium on serum glucose: an assay for biologically active chromium. Nutr Reports Intl,1985;32(1):1-7
[49] Vinson JA, Bose P. The effect of high chromium yeast on the blood glucose control and blood lipids of normal and diabetic human subjects. Nutr Reports Intl, 1984;30(4):911-918
[50] Vinson JA, Howard TB. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. Nutr Biochemistry, 1996;7:659-663
[51] Vinson J. Rat zinc bioavailability study. University of Scranton, Scranton (PA)
[52] Vinson J. Bioavailability of magnesium. University of Scranton, Scranton (PA), 1991
[53] Frequently Asked Questions. www.albionlabs.com July 19, 2002
[54] Rouhi AM. Escorting metal ions: protein chaperone protects, guides, copper ions in transit. Chem Eng News 1999;11:34-35
[55] Himelblau E, et al. Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from Arabidopsis. Plant Physiol 1998;117(4):1227-1234
[56] Lapinskas PJ, Lin SJ, Culotta VC. The role of Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol Microbiol 1996;21(3):519-528
[57] Allen LH, Wood RJ. Calcium and Phosphorus. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:144-163
[58] Heaney RP, Dowell MS, Barger-Lux MJ. Absorption of calcium as the carbonate and citrate salts, with some observations on method. Osteoporosis Int, 1999;9:19-23
[59] Timon S. Mineral Logic: Understanding the Mineral Transport System. Advanced Nutrition Research: Ellicottville (NY),1985
[60] Burger S. Vitamins and Minerals for Health. Wild Rose College of Natural Healing, Calgary,1988
[61] Orlov SN, Li JM, Tremblay J, Hamet P. Genes of intracellular calcium metabolism and blood pressure control in primary hypertension. Semin Nephrol. 1995 Nov;15(6):569-592
[62] Osborne G, et al. Evidence for the relationship of calcium to blood pressure. Nutr Reviews, 1996;54(12):365-381
[63] Yamamoto ME., et al. Lack of blood pressure effect with calcium and magnesium supplementation with adults with high-normal blood pressure results from phase I of the Trials of Hypertension and Prevention (TOHP). Ann Epidem, 1995;5:96-107
[64] Afghani A, Johnson CA. Resting blood pressure and bone mineral content are inversely related in overweight and obese Hispanic women. Am J Hypertens. 2006;19(3):286-292
[65] Knight KB, Keith RE. Effects of oral calcium supplementation via calcium carbonate versus diet on blood pressure and serum calcium in young, normotensive adults. J Opt Nutr, 1994;3(4):152-158
[66] Weaver CM, Heaney R. Calcium. In Modern Nutrition in Health & Disease, 10th ed. Lippincott Williams & Wilkins, Phil., 2006:194-210
[67] Nielson F. Chromium. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:264-268
[68] Hendlor S, Rorvik D, eds. PDR for Nutritional Supplements, 1st ed. Medical Economics, Montvale (NJ), 2001
[69] Turnland JR. Copper. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:231-241
[70] Hetzel BS, Clugston GA. Iodine. In Modern Nutrition in Health and Disease, 9th ed. Lea & Febiger, Phil.,1999:253-264
[71] Greene HL and Moran JR. The Gastrointestinal Tract: Regulation of Nutrient Absorption. In Modern Nutrition in Health and Disease, 8th ed. Lea and Febiger, Phil.,1994:549-568
[72] Fairbanks VF. Iron in Medicine and Nutrition. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:185-213
[73] Shils M. Magnesium. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:164-184
[74] Levander OA, Burk RF. Selenium. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:242-263
[75] Whitaker J. Minerals, part 1: Cut your cancer risk with selenium. Health & Healing, 1999;9(4):6-8
[76] Cunnane SC. Zinc: Clinical and Biochemical Significance. CRC Press, Boca Raton (FL),1988
[77] Bauernfeind JC. Nutrification of foods. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:1579-1592
[78] Ghebremeskel K, Crawford MA. Nutrition and health in relation to food production and processing. Nutr Health, 1994;9(4):237-253
[79] Bazzarre TL, Hopkins RG, Wu SM, Murdoch SD. Chronic disease risk factors in vitamin/mineral 9supplement users and nonusers in a farm population. J Am Coll Nutr, 1991;10(3):247-257
[80] Sax NI, Lewis RJ. Hawley’s Condensed Chemical Dictionary, 11th ed. Van Nostrand Rheinhold, New York,1987
[81] Burr-Madsen A. Gateways College of Natural Therapies, Module 1. Gateway College, Shingle Springs (CA), 1996
[82] Erdman JW, Poneros-Schneir AG. Factors affecting the nutritive value in processed foods. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:1569-1578
[83] Ascherio A and Willett WC. Health effects of trans fatty acids. Am J Clin Nutr, 1997;66:1006S-1010S
[84] Hornick SB. Factors affecting the nutritional quality of crops. AM J Alternative Ag,1992;7(1-2)
[85] Organic tomatoes, vitamin C, and calcium. Nutr Week, 1998;28(24):7
[86] Smith BL. Organic foods vs. supermarket foods: J Applied Nutr,1993;45(1):35-39
[87] Mursu J., et al. Dietary Supplements and Mortality Rate in Older WomenThe Iowa Women’s Health Study. Arch Intern Med. 2011;171(18):1625-1633
[88] Boland MJ, et al. Calcium Supplements and Cardiovascular Risk. Ther Adv in Drug Safe. 2013;4(5):199-210
Some of these studies (or citations) may not conform to peer review standards. Therefore, the results are not conclusive. Professionals can, and often do, come to different conclusions when reviewing scientific data. None of these statements have been reviewed by the FDA. All products distributed by Doctors’ Research, Inc. are nutritional an
In a nutritional context, minerals are certain elements, such as iron and phosphorus that are essential for the physiology of living organisms to exist.
When it comes to nutrition, plants and humans differ: “a typical plant makes its own food from raw materials… A typical animal eats its food” [1]. For plants, these raw materials include soil-based inorganic mineral salts [2]. Soil-based mineral salts can be depleted through synthetic fertilizers, herbicides, pesticides, as well as repeatedly growing crops on the same soil [3,4].
Plants, with the aid of enzymes and soil-based microorganisms, can take in from soil the mineral salts that they have an affinity for through their roots or hyphae [4]. After various metabolic processes, when these minerals no longer exist as salts, they become complexed with various carbohydrates, lipids, and proteins present in the plant as part of the living organism [5]. Thus for nutrition, humans eat plants and/or animals that eat plants, whereas plants can obtain their nutrients from the soil [4]. This process is commonly referred to as the “food chain” [5].
Unfortunately most mineral supplements contain minerals in the form referred to as ‘mineral salts’. Even though mineral salts are often called “natural”, they are rocks (e.g. calcium carbonate exists as the rock commonly known as limestone) or they are chemically produced in accordance with the United States Pharmacopoeia (USP). Mineral salts are natural food for plants, they are not a natural food for humans–humans do not have roots or hyphae!
Dietary Guideline number 18 of the Weston A. Price Foundation, an organization devoted to consuming real foods, is: “Use only natural, food-based supplements” [6]. One of the standards of naturopathy agreed to in 1947 was, “Naturopathy does not make use of synthetic or inorganic vitamins or minerals” [7]. Why would naturopaths have mentioned minerals since they are ‘natural’? Because even back then, most naturopaths knew that the inorganic minerals being placed into supplements were often simply industrial rocks and not foods. Little has changed in the nearly seven decades since. This paper documents the availability, sources, and some of the chemical differences between minerals found in foods and the industrially processed mineral salts that are found in most ‘natural’ mineral supplements.
Absorption
Mineral absorption is affected by many factors including the chemical form, structural form, existence or lack of protein chaperones, health, dietary factors, and even medications.
“Absorptive efficiency for many minerals is governed by homeostatic feedback regulation. When the body is in a depleted state, the intestine upregulates absorption of the nutrient. At the biochemical level , this regulation must be expressed by the control of intraluminal binding lignans, cell-surface receptors, intracellular carrier proteins, intracellular storage proteins, or the energetics of the transmembrane transport…In general mineral bioavailability decreases because of many drugs, decreases with age, and in the presence of malnutrition, is associated with poorer integrity of the small intestine. Therefore, older individuals who are often taking numerous medications and who are eating more poorly than young people are at greater risk of mineral deficiencies” [8].
Chemical Differences
The basic difference between minerals found in foods and those found in industrial mineral salts is chemical.
“The chemical form of a mineral is an important factor in its absorption and bioavailability…there is evidence that the form in which minerals are ingested affects absorption. For example, particle size, surface area, and solubility of a substance affects is dilution rate…In many solid foods, elements are not free, but firmly bound in the food matrix” [8].
This, of course, is not true of most minerals in supplements as they are normally industrially processed inorganic rocks (mineral salts) hence they are void of the factors found in a food matrix. Only 100% food minerals have minerals attached in a food matrix.
Minerals are normally found in food and in the body they are attached with some peptide [9,10]. When humans eat plants or animals they are consuming minerals in those forms. Humans are not supposed to directly consume soil components [1]. With the exception of sodium chloride (common table salt), humans do not normally in any significant quantity consume minerals in the chemical forms known as mineral salts. When they do, it is considered to be a disorder called ‘geophagia’ or ‘pica’ [11,12].
It is a fact that mineral salts are often called “natural”, but they are not food minerals. Mineral salts are normally inorganic molecular compounds that look like rocks [13]. Mineral salts are a compound containing a mineral element, which is the mineral normally listed on a supplement label, and some other substance it is chemically bound to. Mineral salts are either rocks (e.g. calcium carbonate exists as the rock commonly known as limestone) or they are rocks that are chemically-altered. Mineral salts are natural food for plants which can chemically change and detoxify them [14]; they are not a natural food for humans, although some people do consider crushed bones and naturally-calcified sea algae, etc. as food. Minerals bound in mineral salts simply are not treated the same way in the body as are minerals found in food.
Minerals vs. Industrial Chemicals
The following list describes what many mineral salts/chelates used in supplements actually are and what they are used for when not in supplements:
- Boric acid is the rock known as sassolite. It is used in weatherproofing wood, fireproofing fabrics, and as an insecticide [15].
- Calcium ascorbate is calcium carbonate processed with ascorbic acid and acetone. It is a manufactured product used in ‘non-food’ supplements [15].
- Calcium carbonate is the rock known as limestone or chalk. It is used in the manufacture of paint, rubber, plastics, ceramics, putty, polishes, insecticides, and inks. It is also used in fillers for adhesives, matches, pencils, crayons, linoleum, insulating compounds, and welding rods [15].
- Calcium chloride is calcium carbonate and chlorine and is the by product of the Solvay ammonia-soda process. It is used for antifreeze, refrigeration, fire extinguisher fluids, and to preserve wood and stone. Other uses include cement, coagulant in rubber manufacturing, controlling dust on unpaved roads, freezeproofing of coal, and increasing traction in tires [15].
- Calcium citrate is calcium carbonate processed with lactic and citric acids. It is used to alter the baking properties of flour [15].
- Calcium gluconate is calcium carbonate processed with gluconic acid, which is used in cleaning compounds. It is used in sewage purification and to prevent coffee powders from caking [15].
- Calcium glycerophosphate is calcium carbonate processed with dl-alpha-glycerophosphates. It is used in dentifrices, baking powder, and as a food stabilizer [15].
- Calcium hydroxyapatite is crushed bone and bone marrow. It is used as a fertilizer [16].
- Calcium iodide is calcium carbonate processed with iodine. It is an expectorant [15].
- Calcium lactate is calcium carbonate processed with lactic acid. It is used as a dentifrice and as a preservative [15].
- Calcium oxide is basically burnt calcium carbonate. It is used in bricks, plaster, mortar, stucco, and other building materials. It is also used in insecticides and fungicides [15].
- Calcium phosphate, tribasic is the rock known as oxydapatit or bone ash. It is used in the manufacture of fertilizers, milk-glass, polishing powders, porcelain, pottery, and enamels [15].
- Calcium stearate is an octodecanoic calcium salt and can be extracted from animal fat. It is used for waterproofing fabrics and in the production of cement, stucco, and explosives [15].
- Chromium chloride is a preparation of hexahydrates. It is used as a corrosion inhibitor and waterproofing agent [15].
- Chromium picolinate is chromium III processed with picolinic acid. Picolinic acid is used in herbicides [17].
- Copper aspartate is made “from the reaction between cupric carbonate and aspartic acid (from chemical synthesis)” [18]. It is a manufactured product used in ‘non-food’ supplements [18].
- Copper (cupric) carbonate is the rock known as malachite. It is used as a paint and varnish pigment, plus as a seed fungicide [15].
- Copper gluconate is copper carbonate processed with gluconic acid. It is used as a deodorant [19].
- Copper (cupric) glycinate is a copper salt processed with glycine. It is used in photometric analysis for copper [15].
- Copper sulfate is copper combined with sulfuric acid. It is used as a drain cleaner and to induce vomiting; it is considered as hazardous heavy metal by the City of Lubbock, Texas that “can contaminate our water supply” [20].
- Dicalcium phosphate is the rock known as monetite, but can be made from calcium chloride and sodium phosphate. It is used in ‘non-food’ supplements [18].
- Ferric pyrophosphate is an iron rock processed with pyrophosphoric acid. It is used in fireproofing and in pigments [15].
- Ferrous lactate is a preparation from isotonic solutions. It is used in ‘non-food’ supplements [15].
- Ferrous sulfate is the rock known as melanterite. It is used as a fertilizer, wood preservative, weed-killer, and pesticide [15].
- Magnesium carbonate is the rock known as magnesite. It is used as an antacid, laxative, and cathartic [15].
- Magnesium chloride is magnesium ammonium chloride processed with hydrochloric acid. It fireproofs wood, carbonizes wool, and is used as a glue additive and cement ingredient [15].
- Magnesium citrate is magnesium carbonate processed with acids. It is used as a cathartic [15].
- Magnesium glycinate is a magnesium salt processed with glycine. It is used in ‘non-food’ supplements.
- Magnesium oxide is normally burnt magnesium carbonate. It is used as an antacid and laxative [15].
- Manganese carbonate is the rock known as rhodochrosite. It is used as a whitener and to dry varnish [15].
- Manganese gluconate is manganese carbonate or dioxide processed with gluconic acid. It is a manufactured item used in ‘non-food’ supplements [15].
- Manganese sulfate is made “from the reaction between manganese oxide and sulfuric acid” [18]. It is used in dyeing and varnish production [15].
- Molybdenum ascorbate is molybdenite processed with ascorbic acid and acetone. It is a manufactured item used ‘non-food’ supplements [21].
- Molybdenum disulfide is the rock known as molybdenite. It is used as a lubricant additive and hydrogenation catalyst [15].
- Potassium chloride is a crystalline substance consisting of potassium and chlorine. It is used in photography [15].
- Potassium iodide is made from HI and KHCO3 by melting in dry hydrogen and undergoing electrolysis. It is used to make photographic emulsions and as an expectorant [15].
- Potassium sulfate appears to be prepared from the elements in liquid ammonia. It is used as a fertilizer and to make glass [15].
- Selenium oxide is made by burning selenium in oxygen or by oxidizing selenium with nitric acid. It is used as a reagent for alkaloids or as an oxidizing agent [15].
- Seleniomethionine is a selenium analog of methionine. It is used as a radioactive imaging agent [15].
- Silicon dioxide is the rock known as agate. It is used to manufacture glass, abrasives, ceramics, enamels, and as a defoaming agent [15].
- Vanadyl sulfate is a blue crystal powder known as vanadium oxysulfate. It is used as a dihydrate in dyeing and printing textiles, to make glass, and to add blue and green glazes to pottery [15].
- Zinc acetate is made from zinc nitrate and acetic anhydride. It is used to induce vomiting [15].
- Zinc carbonate is the rock known as smithsonite or zincspar. It is used to manufacture rubber [15].
- Zinc chloride is a combination of zinc and chlorine. It is used as an embalming material [15].
- Zinc citrate is smithsonite processed with citric acid. It is used in the manufacture of some toothpaste [15].
- Zinc gluconate is a zinc rock processed with gluconic acid. Gluconic acid is used in many cleaning compounds [15].
- Zinc lactate is smithsonite processed with lactic acid. Lactic acid lactate is used as a solvent [15].
- Zinc monomethionine is a zinc salt with methionine. It is used as a ‘non-food’ supplement.
- Zinc orotate is a zinc rock processed with orotic acid. Orotic acid is a uricosuric (promotes uric acid excretion) [15].
- Zinc oxide is the rock known as zincite. It is used as a pigment for white paint and as part of quick-drying cement [15].
- Zinc phosphate is the rock known as hopeite. It is used in dental cements [15].
- Zinc picolinate is a zinc rock processed with picolinic acid. Picolinic acid is used in herbicides [17].
- Zinc sulfate can be a rock processed with sulfuric acid. It is used as a corrosive in calico-printing and to preserve wood [15].
There is a relatively easy way to tell if minerals are industrial chemicals. Whenever there are two-words on a label describing a mineral, it is a logical to conclude that the substance is an industrial mineral product and not 100% food. The exception is chromium GTF (the GTF stands for glucose tolerance factor) which is food if it is from nutritional yeast [18].
Chelated Minerals
Chelated minerals are generally crushed industrial rocks that are processed with one or more acids.
Probably the biggest difference in minerals now compared to 1947 is that some companies have decided to industrially produce versions of minerals attached to peptides. Essentially they take a rock or industrial mineral salt, chemically alter it, and attempt to attach it to the mineral. This results in a mineral that is different from normal mineral salts, but does not turn the substance into a food. Examples of this include the various mineral ascorbates, picolinates, aspartates, glycinates, and chelates. It needs to be understood that since there is not a universally accepted definition of the term ‘chelate’, when this term is used on a label, one generally does not know if the chelate is amino-acid based or some type of industrial acid.
While it is true that humans can, and do, utilize minerals from USP mineral salts or chelated minerals, this is not as safe (or even normally as effective) as consuming them from foods (or in the case of real food supplements, food concentrates).
Non-Food Attachments, Including Some “Chelates,” Are Not Desirable
Is it wise to consume non-food minerals?
Dr. Bernard Jensen, an early 20th century advocate of food-based nutrition, once wrote, “When we take out from foods some certain salt, we are likely to alter the chemicals in those foods. When extracted from food, that certain chemical salt is extracted, may even become a poison. Potash by itself is a poison, whether it comes from a food or from the drugstore. This is also the case with phosphorus. You thereby overtax your system, and your functions must work harder, in order to throw off those inorganic salts or poisons introduced… The chemical elements that build our body must be in biochemical, life-producing form. They must come to us as food, magnetically, electrically alive, grown from the dust of the earth… When we are lacking any element at all, we are lacking more than one element. There is no one who ever lacked just one element. We don’t have a food that contains only one element, such as a carrot entirely of calcium or sprouts totally made of silicon” [22].
It should be noted that the addition of “citric acid and picolinic acid do not appear to enhance zinc absorption” [23]. Chromium picolinate is a human-made substance, created by Gary Evans [24]; it is not a natural food. Picolinic acid is used in herbicides [17]; furthermore “picolinic acid is an excretory or waste product. It is not metabolized by or useful to the body” [25]. Scientists report, “some research groups recently suggested that chromium (III) picolinate produces significantly more oxidative stress and potential DNA damage than other chromium supplements” [26].
Concerns are being raised from various sources about the implications of intentional ingestion of inorganic substances in supplements by human beings [22,25,26]. These substances are not natural for humans to consume and a long period of consumption may cause some type of toxic accumulation [22,25,26]. Yet, many people supposedly interested in natural health are daily consuming various carbonates, gluconates, oxides, picolinates, phosphates, sulfates and other rock components that were not intended to be ingested that way. Since there are many possible negative implications associated with “the other half” of these non-food minerals [25], people truly interested in their health would be much better off consuming foods that are high in minerals or supplements made from those foods.
Jay Patrick claims to have originally developed procedures to manufacture all seven of the mineral ascorbates [21]; thus it would seem highly inappropriate to call supplements with ascorbate attached minerals ‘food’.
Actually, it does not appear that any of the minerals marketed as ‘chelated’ are food concentrates, though there are foods which contain naturally chelated minerals, but these are normally marketed as food minerals. Even though there are some theoretical advantages to industrially-produced mineral ‘chelates’ as compared to inorganic mineral salts, these chelates are not natural food.
More on Bioavailability
It is well known among nutrition researchers that most essential minerals are not well absorbed; for some minerals, absorption is less than 1% [27]. “Bioavailability of orally administered vitamins, minerals, and trace elements is subject to a complex set of influences…In nutrition science the term ‘bioavailability’ encompasses the sum of impacts that may reduce or foster the metabolic utilization of a nutrient” [28]. Research demonstrates that the bioavailability and/or effectiveness of mineral containing foods is greater than that of isolated inorganic mineral salts or mineral chelates [e.g. 28-52]. These studies have concluded that natural food minerals may be better absorbed, utilized, and/or retained than mineral salts.
Furthermore, minerals used in most supplements do not contain protein chaperones or other food factors needed for absorption into the cell. In 1999, the Nobel Prize for medicine was awarded to Guenter Blobel who discovered that minerals need protein chaperones to be absorbed into cellular receptors. When mineral salts without protein chaperones are consumed, “It is after digestion when other mineral forms {mineral salts} have their mineral cleaved from their carriers. In this situation, these minerals become charged ions, and their absorbability becomes in jeopardy. These charged free minerals are known to block the absorption of one another, or to combine with other dietary factors to form compounds that are unabsorbable” [53]. The body must discard the residual chemicals.
Foods used in supplements that commonly provide significant quantities of essential minerals include dulse, horsetail herb, kelp, nutritional yeast, rice bran, and water thyme. These types of foods have been shown to contain not only minerals in natural food forms, but also important protein chaperones such as ATX1 and ceruplasmin [54,55]. Industrial mineral salts do not contain the protein chaperones or other food factors needed for proper mineral absorption. Furthermore, some foods also contain factors which reduce the probability of certain minerals to be toxic to the body [32,33,55]; industrial mineral salts and chelates are simply not that complete.
Quantitative and Qualitative Differences
There are quantitative and qualitative differences in food vs. non-food minerals. Table 1 lists some of them by mineral.
Table 1 Quantitative and Qualitative Differences
| Food Mineral | | Compared to Mineral Salt/Chelate |
| Calcium | | Up to 8.79 times more absorbed into the blood [47] and 7 times as effective in raising serum ionic calcium levels [30]. |
| Chromium | | Up to 25 times more bioavailable [31]. |
| Copper | | 85% more absorbed [45]; also contains substances that reduce potential toxicity [32,46]. |
| Iron | | Safer, non-constipating, 77% more absorbed [33, 34, 45]. |
| Magnesium | | Up to 2.20 times better absorbed [52] and retained [35]. |
| Manganese | | Better absorbed and retained [45,46] and not as likely to contribute to toxicity as mined forms [36,56]. |
| Molybdenum | | Up 6.28 times better absorbed into the blood and 16.49 times better retained [45]. |
| Phosphorus | | Less likely to cause diarrhea or electrolyte disorders [37]. |
| Selenium | | 17.6 time the antioxidant effect [46], 123.01 times more effective in preventing nonenzymatic protein glycation [17], and 2.26 times better retained [29,38,44]. |
| Vanadium | | Safer and 50% more effective [39]. |
| Zinc | | Up to 6.46 times better absorbed [45,46,51], better form [40,41]. |
Foods, almost by definition, are not toxic, and as mentioned earlier, can have protective factors to prevent certain potential mineral toxicities, such as those sometimes associated with copper, iron, manganese, or other minerals [32,33,55,56].
Information by Individual Mineral
Some differences between food complexed minerals and mineral salts have been documented by published research and are shown by individual mineral below:
Boron “Boron complexes with organic compounds containing hydroxyl groups” [9], which is how it is found in foods. Boron affects macromineral and steriodal hormone metabolism; without sufficient boron bone composition, strength, and structure weaken [9].
Calcium “The amount of calcium absorbed depends on its interaction with other dietary constituents…The absorbability of calcium is mainly determined by the presence of other food constituents” [56]. This is one of the reasons why isolated calcium mineral salts (such as calcium carbonate) are not absorbed as well as calcium found in natural food complexes [56,57]. “Calcium carbonate, an antacid, counteracts not only the absorption of calcium, but also the absorption of iron” [11] (though its calcium absorption appears to be better with food [58]). At least one researcher has concluded that commonly used mineral salts such as calcium lactate and calcium gluconate primarily succeed in creating high blood calcium levels (hypercalcemia) instead of alleviating symptoms of low tissue calcium [59]. “Calcium has a structural role in bones and teeth” as well as in some enzymes involved with blood clotting [48]. Calcium can affect mood and blood pressure [57,60]. Clinical reports consistently confirm that dietary/food calciums [5-8] are important in the management of blood pressure. This does not appear to be the case with isolated calcium salts (the results appear inconsistent [30,61-63]). One study found that calcium in Food raised serum ionic calcium levels from 1.08 to 1.15 mmoles, but that serum ionic calcium levels were not raised with calcium carbonate [30]. Serum calcium levels affect blood pressure [60,64]. Since low bone mass is somewhat inversely correlated with high levels of diastolic blood pressure [9], this suggests that calcium from Food may be superior when hypertension issues are present. Calcium is important for optimal health as calcium deficiencies can contribute to osteoporosis, muscle cramps (especially in the legs), insomnia, mood/behavioral/nerve problems, hypertension, kidney stones, and colon cancer [61,65,66]. It appears that overdose of calcium can only occur when taking mineral salt forms of calcium supplement as opposed to food [66]. A human study found that Natural Food Complex calcium is 8.79 times more bioavailable than calcium carbonate (which is the most common form found in supplements) and 2.97 times more than calcium gluconate [47]. This same study found that Food calcium “produced no undesirable side effects and was the most suitable form of calcium for long-term supplementation” [47].
Chromium, GTF “The biologically active form of chromium, sometimes called glucose tolerance factor or GTF, has been proposed to be a complex of chromium, nicotinic acid, and possibly the amino acids glycine, cysteine, and glutamic acid. Many attempts have been made to isolate or synthesize the glucose tolerance factor; none have been successful” [67]. Chromium is not naturally found in the body in the commonly supplemented forms such as chromium picolinate or chromium chelate. “Chromium is generally accepted as an essential nutrient that potentiates insulin action, and thus influences carbohydrate, lipid, and protein metabolism” [67]. Research suggests that there is much less likelihood of toxicity from natural food complex chromium than from forms such as chromium picolinate [26]. Only 1% or less of inorganic chromium is absorbed vs.10-25% of chromium GTF [31]. One small study found that Food chromium GTF reduced blood glucose levels by 16.8% versus 6.0% for inorganic chromium [48], thus it was 2.80 times more effective. One study found that Food chromium benefited certain diabetics by improving blood glucose control, lowering serum lipids, and decreasing the risk of coronary heart disease [49]. Chromium GTF only comes from nutritional yeast [58].
Copper In the human body, in addition to various plasma-bound coppers, “at least one copper peptide complex” has been isolated [60]. Copper is predominantly found in Food nutrients in a copper peptide complex (such as Cu/Zn superoxide-dismutase). Copper is not naturally found in the body in the form of copper gluconate or copper sulfate. “Anemia, neutropenia, and osteoporosis are observed with copper deficiency”; copper is involved in connective tissue, iron metabolism, the central nervous system, melanin pigment, thermal regulation, cholesterol metabolism, immune function, and cardiac function [60]. Copper in foods like nutritional yeast contains protective factors that reduce the possibility of toxicity issues [32,46]. A human study found that Food copper was 1.44 times more absorbed into the blood than copper sulfate and 1.43 times more than copper gluconate [45]. Animal studies showed similar results, plus concluded that Food copper was retained in the liver 1.85 times more than copper gluconate and 1.42 times more than copper sulfate [45].
Iodine Most of the iodine in the body exists in the form of iodine-containing amino acids [61]. Iodine is needed by the thyroid gland to produce thyroid hormones which influence most of the body’s metabolic processes [61]. Kelp is an excellent food source of iodine [61].
Iron Most researchers acknowledge that organic iron is better absorbed than inorganic iron [71]. The body has different mechanisms for the absorption of iron depending upon its form [72]. Iron in foods is found in an organic form. Iron is required for growth and hemoglobin formation; inadequate amounts can lead to “weakness, fatigue, pallor, dyspnea on exertion, palpitation, and a sense of being overly tired” [72]. Iron in food is safer, less-constipating (actually it is non-constipating), and better absorbed than non-food forms [33,34]. An animal study found that Food iron was absorbed into the blood 1.01 times more than ferrous sulfate and 1.77 times more than amino acid chelated iron and was retained in the liver 1.21 times more than ferrous sulfate and 1.68 times more than amino acid chelated iron [45,46].
Magnesium “The percentage of absorption of ingested magnesium is influenced by its dietary concentration and by the presence of inhibiting or promoting dietary components [73]. There are no promoting dietary components in inorganic isolated magnesium salts. “Magnesium is involved in many enzymatic steps in which components of food are metabolized and new products are formed”; it is involved in over 300 such reactions [6]. Clinical deficiency of magnesium can results in “depressed tendon reflexes, muscle fasciculations, tremor, muscle spasm, personality changes, anorexia, nausea, and vomiting” [73]. Magnesium in foods is better absorbed and retained than magnesium from inorganic mineral salts [35]. A human study found that Natural Food Complex magnesium was 2.20 times more absorbed into blood than magnesium oxide and 1.60 times more than amino acid chelated magnesium [52].
Manganese In the body, absorbed manganese complexes with various peptides [9]. Manganese is predominantly found in foods in a manganese peptide complex (such as Mn superoxide-dismutase). It is not found in the body in forms like manganese sulfate. Manganese deficiency can cause “impaired growth, skeletal abnormalities, disturbed or depressed reproductive function, ataxia of the newborn, and defects in lipid and carbohydrate metabolism” [9]. It can also affect skin, hair, nails, and problems with calcium metabolism [9]. Manganese in foods is safer and much less likely to cause any toxicity compared to mined forms [36,56]. ]. An animal study found that Natural Food Complex manganese was absorbed 1.56 times more into the blood and was retained 1.63 times more in the liver than manganese sulfate [45,46].
Molybdenum Molybdenum…in foods…is readily absorbed” [9]. “Molydenum in {nearly all} nutritional supplements is in the form of either sodium molybdate or ammonium molybdate. Molybdenum in food is principally in the form of molydenum cofactors” [67]. “Molybdenum functions as an enzyme cofactor”, thus “detoxifies various pyrimidines, purines, pteridines, and related compounds” [9]; it may also affect growth and reproduction [9]. An animal study found that Food molybdenum was absorbed 6.28 times more into the blood and was retained 16.49 times more in the liver than ammonium molybdate and 10.27 times more than molybdenum amino acid chelate [45].
Phosphorus Phosphorus is found in plants [11]. Phosphorus salts can cause diarrhea and other problems [37]—problems that do not happen with phosphorus in foods. Phosphorus works with calcium to produce strong bones [57].
Potassium Potassium is found in plants [11]. Potassium is the leading intracellular electrolyte and is necessary for electrolyte balance, stimulating aldersterone for the adrenal glands, and blood pressure regulation [11]. Dr. Bernard Jensen seemed to believe potassium is only safe in its natural food complex form [22].
Selenium “The predominant form of selenium in animal tissues is selenocysteine” [74]. That is how it is predominantly found in certain foods. One study found that diets naturally high in selenium (daily consumption as high as 724mcg) produced no signs or symptoms of selenium overexposure while another found that exceedingly high consumption of selenium salts could induce selenium poisoning [74]. Selenium seems to support thyroid hormone production, function as part of many enzymes, and have antioxidant effects [74]. Larry Clark, Ph.D. and others have found that selenium in yeast appears to reduce risk of certain cancers [75]. Julian Whitaker, M.D. reports, “The best absorbed form of selenium, and the one used by Dr. Clark’s research, is high-selenium yeast” [75]. A study using 247 mcg/day of high-selenium yeast found that plasma selenium levels were 2-fold higher than baseline values after 3 and 9 months and returned to 136% of baseline after 12 months, whereas there was a 32% increase in blood glutathione levels also seen after 9 months [29]. Food selenium is about twice as well retained as non-food forms [29,38]. Research suggests that Food selenium is 2.26 times more retained in the liver and 1.22 times more absorbed in the blood than sodium selenite [44]. An in vitro study found that Food selenium had 17.6 times the antioxidant effect than did selenomethionine [44]. One study found that Food selenium was 123.01 times more effective than sodium selenite in preventing nonenzymatic glycation in diabetics [50].
Silicon “In animals, silicon is found both free and bound” [9]. Silicon absorption is quite dependent upon the form [9]. Silicon is involved in bone calcification and connective tissue formation [9]. It is also needed for healthy hair and skin [51]. Silicon is found in foods in an organic form.
Trace Minerals Trace minerals, including “ultra trace minerals” are necessary for the proper functioning of human health [9,51]. There are many in the human body, some of which are known to be essential and others of which their “essentialness” is under investigation. Sea vegetables and certain yeasts are a good source of trace minerals [11,31,61].
Vanadium “Vanadate forms compounds with other biological substances” [9]. “Vanadium has been postulated to play a role in the regulation of (NaK)-ATPase, phosphoryl transferase enzymes, adenylate cyclase, and protein kinases; as an enzyme cofactor in the form of vandyl and in hormone, glucose, lipid, and tooth metabolism” [9]. Vanadium in foods is found in an organic form. Vanadium in food is safer than non-food forms and also appears to be about 50% more effective [39].
Zinc Most researchers acknowledge that organic zinc is better absorbed than inorganic zinc [71]. Zinc itself is generally found in the human body in ionic form [71,76]; it is often bound with albumin [23,76] or alpha2-macroglobulin [23] or exists as part of one of the many zinc metalloenzymes [23,76]. Zinc is predominantly found in foods as zinc peptide complex (such as that complexed with superoxide dismutase). Zinc is not naturally found in the body as zinc gluconate, zinc orotate, zinc sulfate, nor zinc picolinate. In humans “zinc deficiency does not exist without deficiency of other nutrients” [76]. Zinc deficiency in humans can cause alopecia, impotence, skin problems, immune deficiencies, night blindness, impaired taste, delayed wound healing, impaired appetite, photophobia, difficulty in dark adaptation, growth retardation, and male infertility [23]. Zinc in yeast-containing foods is better absorbed and is a better form for humans than inorganic forms [40,41]. Studies indicate that Food zinc appears to be 1.72-1.75 times more absorbed in the blood than zinc sulfate (1.71 times more than zinc chelate; 6.46 times more than zinc gluconate; 3.11 times more than zinc orotate) and 1.75-1.87 times more retained in the liver than zinc sulfate (1.45 times more than zinc amino acid chelate; 3.68 times more than zinc gluconate; 1.50 times more than zinc orotate) [45,46,51].
Food and Food Processing
“In the historic struggle for food, humans ate primarily whole foods or so-called natural foods, which underwent little processing…The nutrient content of food usually decreases when it is processed” [77]. “Intensive animal rearing, manipulation of crop production and food processing have altered the qualitative and quantitative balance of nutrients of food consumed by Western society. This change, to which the physiology and biochemistry of man may not be presently adapted to, is thought to be responsible for the chronic diseases that are rampant in the Industrialized Western Countries” [78]. Some reports suggest that simply taking a synthetic multi-vitamin/mineral formula does not change this [79,80].
Dr. Burr-Madsen has written,
Nutrition ‑ in its most basic sense the process by which the organism finds, consumes,liberates, absorbs, and utilizes the nutrients it must have to live. Although food and therefore nutrients are seemingly plentiful, because of modern use of chemical herbicides and pesticides as well as poor air quality and bad water, the nutrients we buy in the market are very inferior. Human bodies require nutrition found in the form of plants, meat, milk, eggs and water, but all animals get their food directly or indirectly from plants, and all plants get their food from the soil. Therefore mineral deficient soil may be one of the greatest original sources of disease in the world today.
Real soil
We cannot appreciate enough the importance of our relationship with the land, with soil. This is particularly so in this era of artificial chemicals, artificial foods, and the abundance of artificial materials on which we have come to depend. This system cannot replace real soil and the living food crops it produces. Our dependence on artificial, manmade products interferes with our relationship with the soil and the natural world in general. Because of this Nutritional supplementation is necessary.
Soil condition.
After genetics and weather, the condition of the soil is the most important factor in thenutrient content of any plant food and, indirectly, of animal foods. The soils of the world have suffered, and continue to suffer, at the hands of farming. The present food production system, while correcting some abuses of the past, inflicts on the soil a variety of new and old insults that diminish its nutrient value. Because of intensive farming, poor crop management, erosion, commercial fertilization, the use of pesticides, and other problematic factors, much of the soil in which our crops are now raised has been depleted, particularly of essential minerals.
The Human Food Chain.
The human food chain includes animals, animal products and plants, which depend directly or indirectly on the soil. Plants draw their nutrients and general health from a complex of inorganic and organic factors. Inorganic substances include oxygen and carbon, nitrogen, phosphorus, and potassium, along with iron, calcium, and an array of other minerals. The chief organic factors range from decaying plant material and animal wastes to earthworms and an amazing variety of microscopic organisms including bacteria, fungi, algae, and protozoa (Hall 1976: 134). All of these elements are important to the health and nutrient value of the crop ‑ and of the animals that feed on it.
Healthy soil.
Healthy soil is America’s greatest natural resource. But few realize that the current state of wide spread soil erosion in North America threatens our way of life. It may be hard to believe, but only a few inches of topsoil stand between you, me, and starvation. We cannot appreciate enough the importance of our relationship with the land, with soil. What is popularly called topsoil is the rich, nutrient‑laden cover of the Earth’s crust from which food crops draw their sustenance. Underneath the topsoil there may be clay, shale, or rock ‑ Substances that do not support food crops. It is only in the precious shallow topsoil that plants are seeded, germinated, sprouted, nurtured, and grown. These plants serve as food for animals on the lowest ends of the food chain. Animals that eat these plants supply food to animals on the highest ends of the food chain. Attention is important because topsoil is easily exhausted from lack of care. The best farmers replenish the soil as it is farmed. Unfortunately, this practice has become an exception to the rule, this is particularly so today.
Depleted Soil.
When the soil becomes depleted, the plants often show symptoms of poor nutrition, much like human deficiency diseases. For example, a general yellow or pale green color (chlorosis) indicates a lack of sulfur and nitrogen and a white or pale‑yellow color iron deficiency. Some of these deficiencies are apparent enough to hurt the marketability of the crop. Most, however, are not visible to the shopper’s or even the farmer’s eye, and the crop is shipped to market deficient as it is. The toll that fertilization and pesticides take on the soil is wide‑reaching, ultimately including the kind of soil erosion that is now plaguing the Midwest. The most direct and immediate loss are the mineral and vitamin deficiencies in the soil that are passed up the food chain to humans (it is a domino effect) [81].
Commercial food processing definitely reduces the nutrient content of food [81, 82] and can be dangerous to human health [83]. The refining of whole grains (including wheat, rice, and corn) has resulted in a dramatic reduction of their natural food complex nutrition [11,82]; specifically the milling of wheat to white flour reduces the natural food complex vitamin and mineral content by 40-60% [82]. Food refining appears to reduce trace minerals such as manganese, zinc, and chromium [2] and various macrominerals (such as magnesium) as well [10,56]. The treatment of canned or frozen vegetables with ethylenediaminetetraacetic acid (EDTA) can strip much of the zinc from foods [11]. The high incidences of disorders of calcium metabolism [28] suggest that the forms of calcium many are consuming simply do not agree with the body (and sometimes result in calcium loss [11]).
Organically-grown produce appears to contain higher levels of some essential minerals than does conventionally (non-organically) grown produce [84,85] and appears to contain lower levels of toxic heavy metals [86]. Even if modern food practices did not affect nutrition (which they do), all minerals that humans need for optimal health do not exist uniformly in soils. “Soils in many areas of the world are deficient in certain minerals; this can result in low concentrations of major or trace minerals in drinking water, plant crops, and even tissues of farm animals, thus contributing to marginal or deficient dietary intakes of humans [76]. From a geological perspective, a few examples include iodine, molybdenum, cobalt, selenium, and boron [2,70,77]. Although humans need at least twenty minerals (over sixty have been found in the body), most plants can be grown with only the addition of nitrogen, phosphorus, and potassium compounds [2]. If other minerals necessary for human health are reduced in the soil, the plant can (and will) grow without them. This means, though, that constantly farming the same ground can result in the reduction of some of the essential minerals we as humans require for optimal health [78].
Ground Up Rocks Pose Risks
Rock minerals are not optimal for human health and post health risks. Perhaps it should be mentioned that typical multi-vitamin-mineral formulas are dangerous and do not result in optimal health. A study involving 38,772 women in the USA who took synthetic multi-vitamins with ground up rock minerals found that the women died earlier than those who did not take them [87]. Other studies have concluded that the acid-processed rocks that many take as calcium supplements increase risk of cardiovascular disease and other problems [88]—yet those studies did not find problems with food calcium.
Ground-up rocks are dangerous to ingest. Yet, 100% food vitamins and minerals are beneficial as well as essential to human health and longevity.
Conclusion
No matter how many industrially produced mineral supplements one takes orally, they will:
1) Never be a truly complete nutrient source.
2) Never replace all the functions of food minerals.
3) Always be unnatural substances to the body.
4) Always strain the body by requiring that it detoxify or somehow dispose of their unnatural structures/chemicals.
5) Never be utilized, absorbed, and retained the same as food nutrients.
6) Not be able to prevent advanced protein glycation end-product formation the same as food nutrients.
7) Never be able to have the antioxidant effects the same as food nutrients.
8) Always be industrial products.
9) Always be composed of petroleum-derivatives, hydrogenated sugars, acids, and/or industrially-processed rocks.
10) Never build optimal health the same as food nutrients.
Industrially processed minerals can have some positive nutritional effects, yet they are not food for humans, but they also pose risks [87-88].
Unlike humans, plants have roots or hyphae which aid in the absorption of minerals. Plants actually have the ability to decrease the toxicity of compounds by changing their biochemical forms [14]. Plants are naturally intended to ingest rocks; humans are not [1].
The truth is that plants, or supplements only made from plants, are the best form of mineral supplement for humans, yet most people who take nutritional mineral support consume some type of industrially processed rock.
REFERENCES
[1] Cronquist A. Plantae. In Synopsis and Classification of Living Organisms, Vol 1. McGraw-Hill, NY, 1982:57
[2] Schroeder HA. The Trace Elements and Man. Devin-Adair, New Greenwich (CT), 1973
[3] Howell E. Enzyme Nutrition. Avery Publishing, Wayne (NJ), 1985
[4] Milne L, Milne M. The Arena of Life: The Dynamics of Ecology. Natural History Press, Garden City (NJ), 1972
[5] Wallace RA. Biology: The World of Life, 6th ed. Harper Collins, New York, 1992
[6] Dietary guidelines in The Weston A. Price Foundation brochure. Weston A. Price Foundation, Washington, 1999
[7] Gehman JM. From the Office of the President: Pseudo-Group Once Again Misleading the Naturopathic Field. Official Bulletin ANA, January 25, 1948:7-8
[8] Shapes SA, Schlussel YR, Cifuentes M. Drug-Nutrient Interactions That Affect Mineral Status. In Handbook of Drug-Nutrient Interactions. Humana Press, Totowa (NJ), 2004: 301-328
[9] Nielsen F. Ultratrace Minerals. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:269-286
[10] Turnland JR. Copper. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:231-241
[11] Whitney EN, Hamilton EMN. Understanding Nutrition, 4ed. West Publishing, New York, 1987
[12] Beers MH, Berkow R, eds. The Merck Manual of Diagnosis and Therapy, 17th ed. Merck Research Laboratories, Whitehouse Station (NJ), 1999
[13] Thiel RJ. Mineral salts are for plants, food complexed minerals are for humans. ANMA Monitor 1999;3(2):5-10
[14] Huang Y, Chen Y, Tao S. Effect of rhizospheric environment of VA-mycorrhizal plants on forms of Cu, Zn, PB and Cd in polluted soil. Ying Yong Sheng Tai Xye Bao 2000;11(3):431-434
[15] Budvari S, et al eds. The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th ed. Merck Research Laboratories, Whitehouse Station (NJ), 1996
[16] Anagisawa KY, Rendon-Angeles JC, Shizawa NI, Ishi SO. Topotaxial replacement of chlorapatite by hydroxy during hydrothermal ion exchange. Am Mineralogist 1999;84:1861-1869
[17] DiTomaso JM. Yellow starthistle: chemical control. Proceedings of the CalEPPC Symposium, 1996, as updated 5/2/02
[18] Vitamin-Mineral Manufacturing Guide Nutrient Empowerment, volume 1. Nutrition Resource, Lakeport (CA), 1986
[19] Hojo Y, Hashimoto I, Miyamoto Y, Kawazoe S, Mizutani T. In vivo toxicity and glutathione, ascorbic acid, and copper level changes induced in mouse liver and kidney by copper (II) gluconate, a nutrient supplement. Yakugaku Zasshi 2000;120(3):311-314
[20] City of Lubbock. www.solidwaste.ci.lubbock.tx.us/hhw/hhw.htm 7/18/02
Cunnane SC. Zinc: Clinical and Biochemical Significance. CRC Press, Boca Raton (FL),1988
[21] Patrick J. What most people don’t know about vitamin C. The Alacer Health Report, Foothill Ranch (CA), 1994
[22] Jensen B. The Chemistry of Man. Bernard Jensen, Escondido (CA),1983
[23] King JC, Keen CL. Zinc. In Modern Nutrition in Health and Disease, 9th ed. Williams & Wilkins, Balt., 1999:223-239
[24] Chromium picolinate, rev. 6/96B.BLI website, July 16, 2002
[25] Implications of the ‘other half’ of a mineral compound. Albion Research Notes 2000;9(3):1-5
[26] Stoecker B.J. Chromium. In Modern Nutrition in Health and Disease, 10th ed. Lippincott Williams & Wilkins, Phil., 2005: 332-337
[27] Turnland JR. Bioavailability of dietary minerals to humans: the stable isotope approach. Crit Rev Food Sci Nutr 1991;30(4);387-396
[28] Schumann K, et al. Bioavailability of oral vitamins, minerals, and trace minerals in perspective. Arzneimittelforshcung 1997;47(4):369-380
[29] El-Bayoumy K, Richie JP Jr, Boyiri T, Komninou D, Prokopczyk B, Trushin N, Kleinman W, Cox J, Pittman B, Colosimo S. Influence of Selenium-Enriched Yeast Supplementation on Biomarkers of Oxidative Damage and Hormone Status in Healthy Adult Males: A Clinical Pilot Study. Cancer Epidemiol Biomarkers Prev. 2002;11:1459-1465
[30] Hamet P, et al. The evaluation of the scientific evidence for a relationship between calcium and hypertension. J Nutr, 1995;125:311S-400S
[31] Ensminger AH, Ensminger ME, Konlade JE, Robson JRK. Food & Nutrition Encyclopedia, 2nd ed. CRC Press, New York, 1993
[32] Avery SV, Howlett NG, Radice S. Copper toxicity towards Saccharomyces cerevisiae: dependence on plasma fatty acid composition. Appl Environ Microbiol 1996;62(11):3960-3966
[33] Wi’snicka R, Krzepiko A, Krawiec Z, Bili’nski T. Protective role of superoxide dismutase in iron toxicity in yeast. Biochem Mol Biol Int 1998;44(3):635-641
[34] Wood R.J., Ronnenberg A.G. Iron. In Modern Nutrition in Health and Disease, 10th ed. Lippincott William & Wilkins, Phil, 2006: 248-270
[35] Rude R.K., Shils M.E. Magnesium. In Modern Nutrition in Health and Disease, 10th ed. Lippincott William & Wilkins, Phil, 2006: 223-247
[36] Buchman A. Manganese. In Modern Nutrition in Health & Disease, 10th ed. Lippincott William & Wilkins, Phil, 2006:326-331
[37] Beloosesky Y, Grinblat J, Weiss A, Grosman B, Gafter U, Chagnac A. Electrolyte disorders following oral sodium phosphate administration for bowel cleansing in elderly patients. Arch Intern Med. 2003;163(7):803-808
[38] Biotechnology in the Feed Industry. Nottingham Press, UK, 1995: 257-267
[39] Badmaev V, Prakash S, Majeed M. Vanadium: a review of its potential role in the fight against diabetes. J Altern Complement Med. 1999;5(3):273-291
[40] Andlid TA, Veide J, Sandberg AS. Metabolism of extracellular inositol hexaphosphate (phytate) by Saccharomyces cerevisiae. Int J. Food Microbiology. 2004;97(2):157-169
[41] King JC, Cousins RJ. Zinc. In Modern Nutrition in Health and Disease, 10th ed. Lipponcott Williams & Wilkins, Phil., 2005:271-285
[42] Thiel R, Fowkes S. Can cognitive deterioration associated with Down syndrome be reduced? Med Hypo, 2005; 64(3):524-532
[43] Jenkins DJA, Wolever TMS, and Jenkins AL. Diet Factors Affecting Nutrient Absorption and Metabolism. In Modern Nutrition in Health and Disease, 8th ed. Lea and Febiger, Phil.:583-602, 1994
[44] Vinson, J.A., Jennifer M. Stella, J.M., Flanagan, T.J. Selenium yeast is an effective in vitro and in vivo antioxidant and hypolipemic agent in normal hamsters. Nutritional Research, 1998, Vol 18, No. 4: 735–742
[45] Vinson J, Bose P, Lemoine L, Hsiao KH. Bioavailability studies. In Nutrient Availability: Chemical and Biological Aspects. Royal Society of Chemistry, Cambridge (UK) 1989:125-127
[46] Vinson JA, Bose P. Comparison of bio-availability of trace elements in inorganic salts, amino acid chelates, and yeast. Mineral Elements 80, Proceedings II, Helsinki, Dec 9-11, 1981
[47] Vinson J, Mazur T, Bose P. Comparisons of different forms of calcium on blood pressure of normotensive males. Nutr Reports Intl, 1987;36(3):497-505
[48] Vinson JA, Hsiao, KH. Comparative effect of various forms of chromium on serum glucose: an assay for biologically active chromium. Nutr Reports Intl,1985;32(1):1-7
[49] Vinson JA, Bose P. The effect of high chromium yeast on the blood glucose control and blood lipids of normal and diabetic human subjects. Nutr Reports Intl, 1984;30(4):911-918
[50] Vinson JA, Howard TB. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. Nutr Biochemistry, 1996;7:659-663
[51] Vinson J. Rat zinc bioavailability study. University of Scranton, Scranton (PA)
[52] Vinson J. Bioavailability of magnesium. University of Scranton, Scranton (PA), 1991
[53] Frequently Asked Questions. www.albionlabs.com July 19, 2002
[54] Rouhi AM. Escorting metal ions: protein chaperone protects, guides, copper ions in transit. Chem Eng News 1999;11:34-35
[55] Himelblau E, et al. Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from Arabidopsis. Plant Physiol 1998;117(4):1227-1234
[56] Lapinskas PJ, Lin SJ, Culotta VC. The role of Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol Microbiol 1996;21(3):519-528
[57] Allen LH, Wood RJ. Calcium and Phosphorus. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:144-163
[58] Heaney RP, Dowell MS, Barger-Lux MJ. Absorption of calcium as the carbonate and citrate salts, with some observations on method. Osteoporosis Int, 1999;9:19-23
[59] Timon S. Mineral Logic: Understanding the Mineral Transport System. Advanced Nutrition Research: Ellicottville (NY),1985
[60] Burger S. Vitamins and Minerals for Health. Wild Rose College of Natural Healing, Calgary,1988
[61] Orlov SN, Li JM, Tremblay J, Hamet P. Genes of intracellular calcium metabolism and blood pressure control in primary hypertension. Semin Nephrol. 1995 Nov;15(6):569-592
[62] Osborne G, et al. Evidence for the relationship of calcium to blood pressure. Nutr Reviews, 1996;54(12):365-381
[63] Yamamoto ME., et al. Lack of blood pressure effect with calcium and magnesium supplementation with adults with high-normal blood pressure results from phase I of the Trials of Hypertension and Prevention (TOHP). Ann Epidem, 1995;5:96-107
[64] Afghani A, Johnson CA. Resting blood pressure and bone mineral content are inversely related in overweight and obese Hispanic women. Am J Hypertens. 2006;19(3):286-292
[65] Knight KB, Keith RE. Effects of oral calcium supplementation via calcium carbonate versus diet on blood pressure and serum calcium in young, normotensive adults. J Opt Nutr, 1994;3(4):152-158
[66] Weaver CM, Heaney R. Calcium. In Modern Nutrition in Health & Disease, 10th ed. Lippincott Williams & Wilkins, Phil., 2006:194-210
[67] Nielson F. Chromium. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:264-268
[68] Hendlor S, Rorvik D, eds. PDR for Nutritional Supplements, 1st ed. Medical Economics, Montvale (NJ), 2001
[69] Turnland JR. Copper. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:231-241
[70] Hetzel BS, Clugston GA. Iodine. In Modern Nutrition in Health and Disease, 9th ed. Lea & Febiger, Phil.,1999:253-264
[71] Greene HL and Moran JR. The Gastrointestinal Tract: Regulation of Nutrient Absorption. In Modern Nutrition in Health and Disease, 8th ed. Lea and Febiger, Phil.,1994:549-568
[72] Fairbanks VF. Iron in Medicine and Nutrition. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:185-213
[73] Shils M. Magnesium. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:164-184
[74] Levander OA, Burk RF. Selenium. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:242-263
[75] Whitaker J. Minerals, part 1: Cut your cancer risk with selenium. Health & Healing, 1999;9(4):6-8
[76] Cunnane SC. Zinc: Clinical and Biochemical Significance. CRC Press, Boca Raton (FL),1988
[77] Bauernfeind JC. Nutrification of foods. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:1579-1592
[78] Ghebremeskel K, Crawford MA. Nutrition and health in relation to food production and processing. Nutr Health, 1994;9(4):237-253
[79] Bazzarre TL, Hopkins RG, Wu SM, Murdoch SD. Chronic disease risk factors in vitamin/mineral 9supplement users and nonusers in a farm population. J Am Coll Nutr, 1991;10(3):247-257
[80] Sax NI, Lewis RJ. Hawley’s Condensed Chemical Dictionary, 11th ed. Van Nostrand Rheinhold, New York,1987
[81] Burr-Madsen A. Gateways College of Natural Therapies, Module 1. Gateway College, Shingle Springs (CA), 1996
[82] Erdman JW, Poneros-Schneir AG. Factors affecting the nutritive value in processed foods. In Modern Nutrition in Health and Disease, 8th ed. Lea & Febiger, Phil.,1994:1569-1578
[83] Ascherio A and Willett WC. Health effects of trans fatty acids. Am J Clin Nutr, 1997;66:1006S-1010S
[84] Hornick SB. Factors affecting the nutritional quality of crops. AM J Alternative Ag,1992;7(1-2)
[85] Organic tomatoes, vitamin C, and calcium. Nutr Week, 1998;28(24):7
[86] Smith BL. Organic foods vs. supermarket foods: J Applied Nutr,1993;45(1):35-39
[87] Mursu J., et al. Dietary Supplements and Mortality Rate in Older WomenThe Iowa Women’s Health Study. Arch Intern Med. 2011;171(18):1625-1633
[88] Boland MJ, et al. Calcium Supplements and Cardiovascular Risk. Ther Adv in Drug Safe. 2013;4(5):199-210
Some of these studies (or citations) may not conform to peer review standards. Therefore, the results are not conclusive. Professionals can, and often do, come to different conclusions when reviewing scientific data. None of these statements have been reviewed by the FDA. All products distributed by Doctors’ Research, Inc. are nutritional and are not intended for the treatment or prevention of any medical condition.




 Vitamin-Mineral™ 90T $40.00
Vitamin-Mineral™ 90T $40.00 Gluco-Sugar-Balance™ 90C $37.00
Gluco-Sugar-Balance™ 90C $37.00 Metabolic Thyro™ 90T $28.00
Metabolic Thyro™ 90T $28.00 B Stress Complex™ 90C $40.00
B Stress Complex™ 90C $40.00
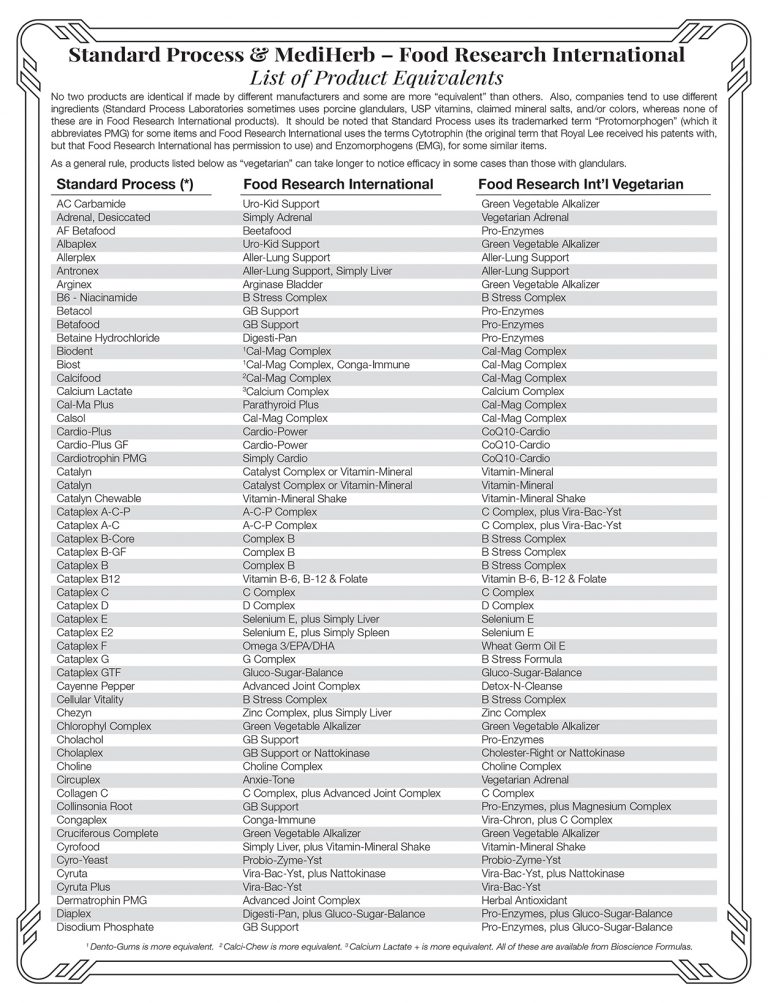




























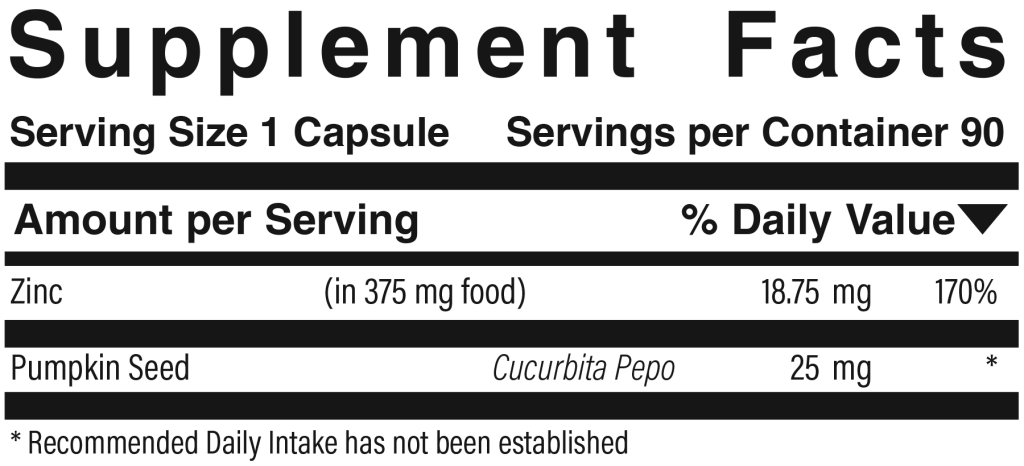








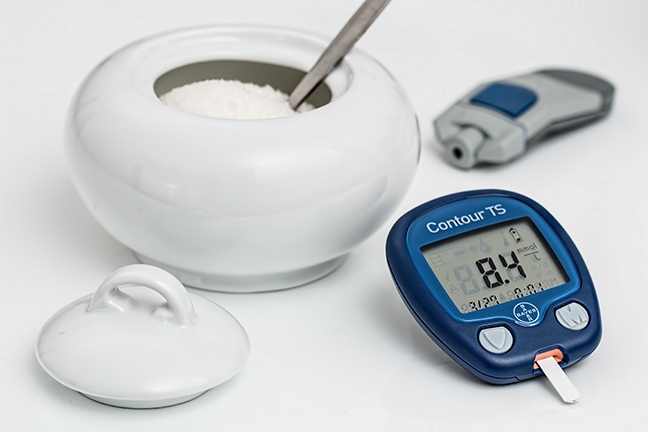


 Food Vitamin C
Food Vitamin C Ascorbic Acid
Ascorbic Acid Food Vitamin B1
Food Vitamin B1 Thiamine Hydrochloride
Thiamine Hydrochloride